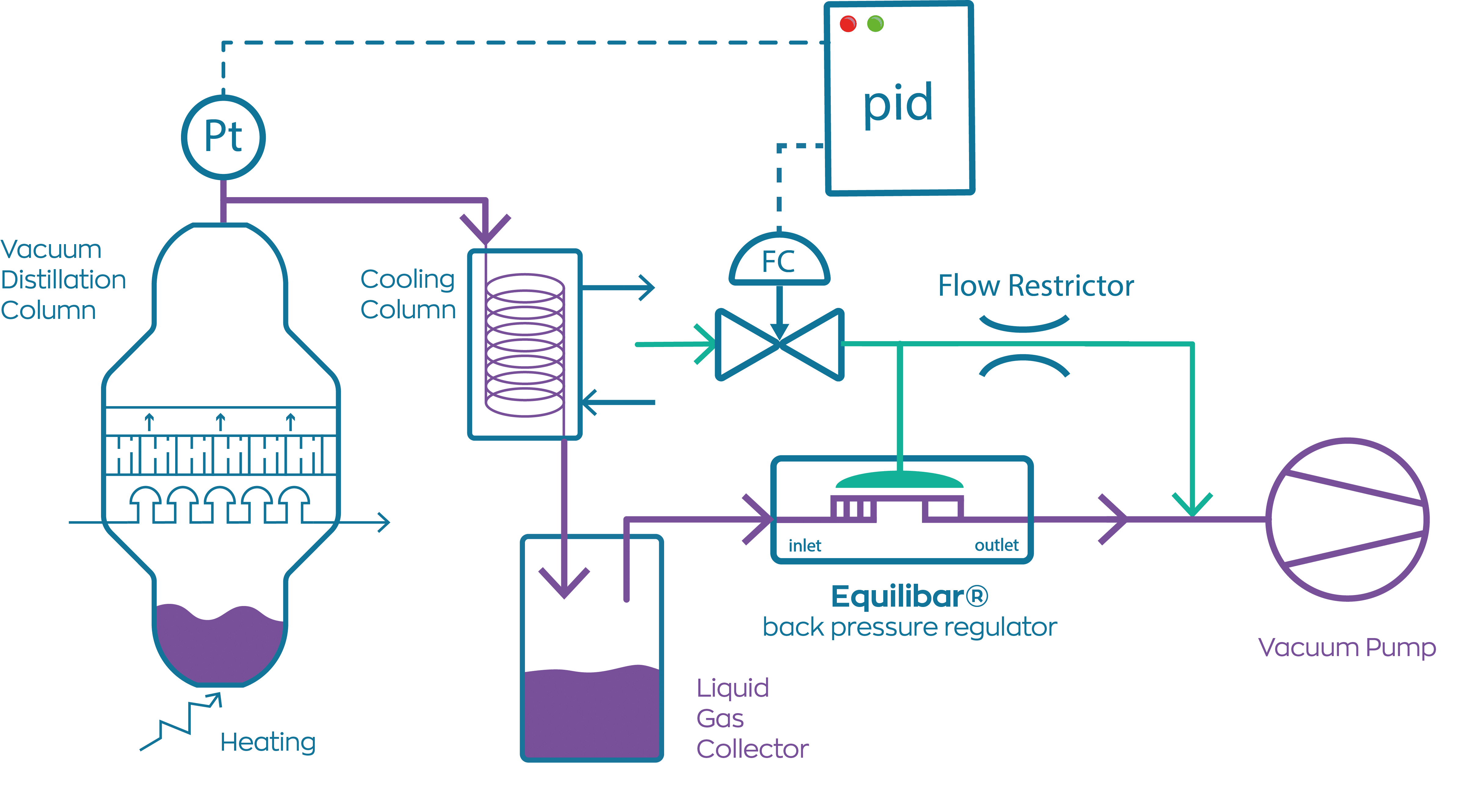
Vacuum Distillation Def . This is performed by lowering the pressures in the column or the reactor. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). In other words, distillation under reduced pressure is known as vacuum distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from.
from www.pressurecontrolsolutions.com
Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In other words, distillation under reduced pressure is known as vacuum distillation. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. This is performed by lowering the pressures in the column or the reactor. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant.
Vacuum Distillation issues? Call Pressure Control Solutions!
Vacuum Distillation Def A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. In other words, distillation under reduced pressure is known as vacuum distillation. This is performed by lowering the pressures in the column or the reactor. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48).
From www.researchgate.net
The schematic for the vacuum distillation process. Download Vacuum Distillation Def Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. A vacuum distillation is performed. Vacuum Distillation Def.
From www.researchgate.net
The vacuum distillation apparatus scheme. Download Scientific Diagram Vacuum Distillation Def Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. In other words, distillation under reduced pressure is known as vacuum distillation. The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. A vacuum distillation is performed by applying a. Vacuum Distillation Def.
From en.wikipedia.org
Distillation Wikipedia Vacuum Distillation Def This is performed by lowering the pressures in the column or the reactor. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. In other words, distillation under reduced pressure is known as vacuum distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150. Vacuum Distillation Def.
From www.pressurecontrolsolutions.com
Vacuum Distillation issues? Call Pressure Control Solutions! Vacuum Distillation Def The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. Boiling commences when the vapor pressure of a liquid. Vacuum Distillation Def.
From eureka.patsnap.com
Vacuum distillation device for indium and distillation method thereof Vacuum Distillation Def A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. This is performed by lowering the pressures in the column or the reactor. The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. In other words, distillation under reduced pressure is known as vacuum distillation. A. Vacuum Distillation Def.
From www.mdpi.com
Chemistry Free FullText New Materials and Phenomena in Membrane Vacuum Distillation Def A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Vacuum distillation is. Vacuum Distillation Def.
From www.thoughtco.com
What Is Distillation? Principles and Uses Vacuum Distillation Def The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o. Vacuum Distillation Def.
From www.vecteezy.com
Distillation, distillation under reduced pressure, vacuum distillation Vacuum Distillation Def Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). The principles of vacuum. Vacuum Distillation Def.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Vacuum Distillation Def A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. This is performed by lowering the pressures in the column or the reactor. In other words, distillation under reduced pressure is known as vacuum distillation. The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. A. Vacuum Distillation Def.
From www.researchgate.net
Diagram of the vacuum distillation still used for purification Vacuum Distillation Def Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in. Vacuum Distillation Def.
From www.pinterest.com
The Various Types of Distillation That are Worth Knowing Distillation Vacuum Distillation Def A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. In other words, distillation under reduced pressure is known as vacuum distillation. This is performed by lowering the pressures. Vacuum Distillation Def.
From www.aquaportail.com
Distillation définition et explications AquaPortail Vacuum Distillation Def This is performed by lowering the pressures in the column or the reactor. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. The principles of vacuum distillation resemble those of fractional. Vacuum Distillation Def.
From www.pinterest.com
Related image Vacuums, Distillation, Education supplies Vacuum Distillation Def The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. This is performed by lowering the pressures in the column or the reactor. A vacuum distillation is used when the boiling point of the compound (or. Vacuum Distillation Def.
From www.mdpi.com
Applied Sciences Free FullText Validation of Diesel Fraction Vacuum Distillation Def A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Vacuum distillation is a. Vacuum Distillation Def.
From www.britannica.com
distillation summary Britannica Vacuum Distillation Def In other words, distillation under reduced pressure is known as vacuum distillation. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Boiling commences when. Vacuum Distillation Def.
From www.geeksforgeeks.org
Methods of Purification of Organic Compounds Vacuum Distillation Def Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. This is performed by lowering the pressures in the column or the reactor. A vacuum distillation is used when the boiling. Vacuum Distillation Def.
From www.laboao.com
Vacuum Distillation Tester Distillation Apparatus China Vacuum Vacuum Distillation Def Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. A distillation operation that is performed at a pressure below. Vacuum Distillation Def.
From www.sme.in
Techpert Process Industries Vacuum Distillation Def A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound. Vacuum Distillation Def.
From proper-cooking.info
Fractional Distillation Setup With Label Vacuum Distillation Def This is performed by lowering the pressures in the column or the reactor. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is used when the boiling point of the compound (or the. Vacuum Distillation Def.
From safrole.com
Vacuum distillation Safrole Vacuum Distillation Def This is performed by lowering the pressures in the column or the reactor. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. Vacuum distillation is a technique for the purification or separation of liquids or solvents. Vacuum Distillation Def.
From www.alamy.com
Vacuum Distillation System Stock Vector Image & Art Alamy Vacuum Distillation Def A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. This is performed by lowering the pressures in the column or the reactor. The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. Vacuum distillation is a technique for the purification or separation of liquids or. Vacuum Distillation Def.
From eureka.patsnap.com
Atmospheric vacuum distillation process for light crude oil Eureka Vacuum Distillation Def The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. This is performed. Vacuum Distillation Def.
From naizaklab.com
High Vacuum Distillation units Naizak Lab Vacuum Distillation Def A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Boiling commences when. Vacuum Distillation Def.
From www.lazada.com.ph
Petroleum vacuum distillation device Petroleum vacuum distillation Vacuum Distillation Def A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation is performed. Vacuum Distillation Def.
From mavink.com
Distillation Phase Diagram Vacuum Distillation Def A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. The principles of vacuum. Vacuum Distillation Def.
From www.youtube.com
Distillation Process Vacuum Distillation Process Distillation Vacuum Distillation Def This is performed by lowering the pressures in the column or the reactor. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. A vacuum distillation is performed by applying a vacuum source to the. Vacuum Distillation Def.
From dokumen.tips
(PDF) Improvement of Vacuum Distillation DOKUMEN.TIPS Vacuum Distillation Def The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation to distinguish it from. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A vacuum distillation is. Vacuum Distillation Def.
From omsonslabs.com
Vacuum Distillation Omsons Labs Vacuum Distillation Def This is performed by lowering the pressures in the column or the reactor. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. Vacuum Distillation Def.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] Vacuum Distillation Def A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. This is performed by lowering the pressures in the column or the reactor. The principles of vacuum distillation resemble those of fractional distillation (commonly called atmospheric distillation. Vacuum Distillation Def.
From www.mdpi.com
Applied Sciences Free FullText Validation of Diesel Fraction Vacuum Distillation Def This is performed by lowering the pressures in the column or the reactor. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. Vacuum Distillation Def.
From www.youtube.com
Vacuum Distillation YouTube Vacuum Distillation Def Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. This is performed by lowering the pressures in the column or the reactor. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. A vacuum distillation is used when the boiling point. Vacuum Distillation Def.
From www.scribd.com
Vacuum Distillation Unit Rev0 AVM 130923 PDF Vacuum Distillation Def Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. This is performed by lowering the pressures in the column. Vacuum Distillation Def.
From glossary.periodni.com
Chemistry Glossary Search results for 'distillation' Vacuum Distillation Def A vacuum distillation is performed by applying a vacuum source to the vacuum adapter of either a simple or fractional distillation (figure 5.48). A vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent off) without significant. Vacuum distillation is a. Vacuum Distillation Def.
From supertekglassware.com
Vacuum Distillation Vacuum Distillation Def A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. This is performed by lowering. Vacuum Distillation Def.
From thepetrosolutions.com
Vacuum Distillation Unit in Oil Refinery Vacuum Distillation Def Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or. A distillation operation that is performed at a pressure below atmospheric pressure is called vacuum distillation. Boiling commences when the vapor pressure of a liquid or solution equals the external or applied pressure. The principles of vacuum distillation. Vacuum Distillation Def.