Acid Base Titration Of Hcl And Naoh . Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. A titration is a technique, in which a reagent, called a titrant, of known concentration. Includes kit list and safety instructions. Adding naoh decreases the concentration of h + because of the neutralization. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Compute sample ph at important stages of a titration.
from www.numerade.com
A titration is a technique, in which a reagent, called a titrant, of known concentration. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. Adding naoh decreases the concentration of h + because of the neutralization. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Compute sample ph at important stages of a titration. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00.
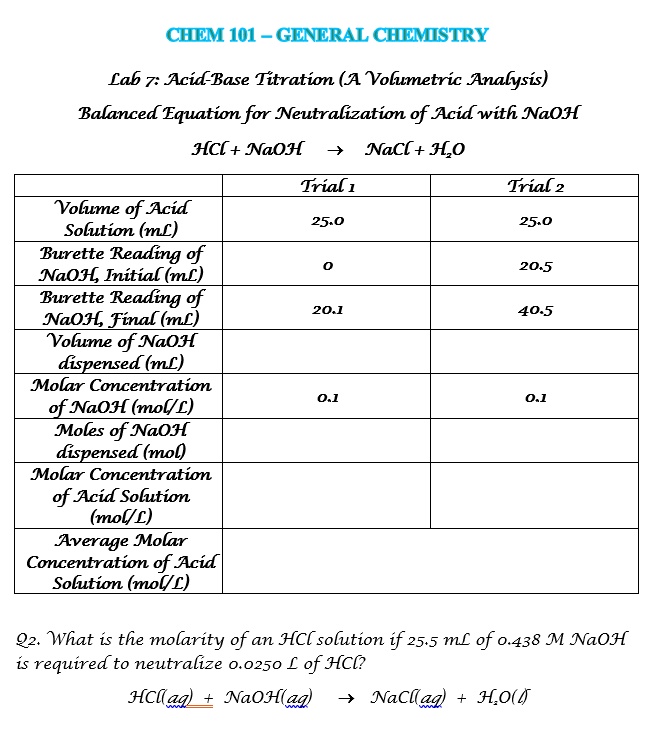
SOLVED CHEM 101 GENERAL CHEMISTRY Lab 7 AcidBase Titration (A
Acid Base Titration Of Hcl And Naoh A titration is a technique, in which a reagent, called a titrant, of known concentration. Compute sample ph at important stages of a titration. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Adding naoh decreases the concentration of h + because of the neutralization. A titration is a technique, in which a reagent, called a titrant, of known concentration. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00.
From mungfali.com
HCl NaOH Titration Acid Base Titration Of Hcl And Naoh A titration is a technique, in which a reagent, called a titrant, of known concentration. Includes kit list and safety instructions. Adding naoh decreases the concentration of h + because of the neutralization. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Because. Acid Base Titration Of Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Acid Base Titration Of Hcl And Naoh Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3. Acid Base Titration Of Hcl And Naoh.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Acid Base Titration Of Hcl And Naoh Includes kit list and safety instructions. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Compute sample ph at important stages of a titration. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A. Acid Base Titration Of Hcl And Naoh.
From www.visionlearning.com
Acids and Bases I Chemistry Visionlearning Acid Base Titration Of Hcl And Naoh Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Adding naoh decreases the concentration of h + because of the neutralization. Includes kit list and safety instructions. Compute sample ph at important stages of a titration. Use this class practical to explore titration, producing. Acid Base Titration Of Hcl And Naoh.
From meetingtarget11.gitlab.io
Ideal Naoh Hcl Balanced Equation What Is The Chemical For Photosynthesis Acid Base Titration Of Hcl And Naoh Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Adding naoh decreases the concentration of h + because of the neutralization. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Because hcl is a. Acid Base Titration Of Hcl And Naoh.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A titration is a technique, in which a reagent, called a titrant, of known concentration. Adding naoh decreases the concentration of h + because of the neutralization. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m. Acid Base Titration Of Hcl And Naoh.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Acid Base Titration Of Hcl And Naoh A titration is a technique, in which a reagent, called a titrant, of known concentration. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Adding naoh decreases the concentration of h + because of the neutralization. Calculate the ph for the strong acid/strong base. Acid Base Titration Of Hcl And Naoh.
From www.youtube.com
Conductometric titration I strong acid (HCl) versus strong base Acid Base Titration Of Hcl And Naoh Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Use this class practical to explore titration, producing the. Acid Base Titration Of Hcl And Naoh.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Acid Base Titration Of Hcl And Naoh Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Adding naoh decreases the concentration of h + because of the neutralization. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Because hcl is a. Acid Base Titration Of Hcl And Naoh.
From www.vrogue.co
What Is The Chemical Equation For Titration Of Hcl Nh vrogue.co Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A titration is a technique, in which a reagent, called a titrant, of known concentration. Includes kit list and safety instructions. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m. Acid Base Titration Of Hcl And Naoh.
From www.numerade.com
SOLVED CHEM 101 GENERAL CHEMISTRY Lab 7 AcidBase Titration (A Acid Base Titration Of Hcl And Naoh A titration is a technique, in which a reagent, called a titrant, of known concentration. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Includes kit list and safety instructions. Adding naoh decreases the concentration of h + because of the neutralization. Compute. Acid Base Titration Of Hcl And Naoh.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration Of Hcl And Naoh Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. A titration is a technique, in which a reagent, called a titrant, of known concentration. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Compute sample. Acid Base Titration Of Hcl And Naoh.
From mungfali.com
Acid Base Titration Procedure Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Compute sample ph at important stages of a titration. Adding naoh decreases the concentration of h +. Acid Base Titration Of Hcl And Naoh.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Acid Base Titration Of Hcl And Naoh A titration is a technique, in which a reagent, called a titrant, of known concentration. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Adding naoh decreases the concentration of h + because of the neutralization. Compute sample ph at important stages of a titration.. Acid Base Titration Of Hcl And Naoh.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Acid Base Titration Of Hcl And Naoh Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Adding naoh decreases the concentration of h + because of the neutralization. A titration is a technique, in which a reagent, called a titrant, of known concentration. Because hcl is a strong acid that. Acid Base Titration Of Hcl And Naoh.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download Acid Base Titration Of Hcl And Naoh Adding naoh decreases the concentration of h + because of the neutralization. Includes kit list and safety instructions. A titration is a technique, in which a reagent, called a titrant, of known concentration. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Compute sample. Acid Base Titration Of Hcl And Naoh.
From mungfali.com
HCl NaOH Titration Acid Base Titration Of Hcl And Naoh A titration is a technique, in which a reagent, called a titrant, of known concentration. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m. Acid Base Titration Of Hcl And Naoh.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Acid Base Titration Of Hcl And Naoh Adding naoh decreases the concentration of h + because of the neutralization. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. A titration is a technique,. Acid Base Titration Of Hcl And Naoh.
From www.pinterest.fr
In our titration lab we used the base NaOH and the acid HCL to create a Acid Base Titration Of Hcl And Naoh Compute sample ph at important stages of a titration. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. A titration is a technique, in which a reagent, called a titrant, of known concentration. Because hcl is a strong acid that is completely ionized. Acid Base Titration Of Hcl And Naoh.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Acid Base Titration Of Hcl And Naoh Compute sample ph at important stages of a titration. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. A titration is a technique, in which. Acid Base Titration Of Hcl And Naoh.
From dokumen.tips
(PDF) AcidBase Titration NaOH with HCL DOKUMEN.TIPS Acid Base Titration Of Hcl And Naoh Adding naoh decreases the concentration of h + because of the neutralization. A titration is a technique, in which a reagent, called a titrant, of known concentration. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Because hcl is a strong acid that. Acid Base Titration Of Hcl And Naoh.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Adding naoh decreases the concentration of h + because of the neutralization. Compute sample ph at important stages of a titration. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the. Acid Base Titration Of Hcl And Naoh.
From www.youtube.com
Acid/Base Neutralization Reaction for NaOH + HCl (Sodium hydroxide Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. A titration is a technique, in which a reagent, called a titrant, of known concentration. Compute sample. Acid Base Titration Of Hcl And Naoh.
From www.youtube.com
Titration of HCl with NaOH YouTube Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Compute sample ph at important stages of a titration. Calculate the ph for the strong acid/strong base. Acid Base Titration Of Hcl And Naoh.
From vdocuments.mx
Acid and Base Titrations MhChemPage I38 / Acid and Base Titrations Acid Base Titration Of Hcl And Naoh Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Includes kit list and safety instructions. A titration is a technique, in which a reagent, called a. Acid Base Titration Of Hcl And Naoh.
From mungfali.com
Titration Curve HCl And NaOH Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Compute sample ph at important stages of a titration. A titration is a technique, in which. Acid Base Titration Of Hcl And Naoh.
From www.youtube.com
Acid Base Equilibria Weak Acid Strong Base Titration. YouTube Acid Base Titration Of Hcl And Naoh Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Adding naoh decreases the concentration of h + because of the. Acid Base Titration Of Hcl And Naoh.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Of Hcl And Naoh A titration is a technique, in which a reagent, called a titrant, of known concentration. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium. Acid Base Titration Of Hcl And Naoh.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Includes kit list and safety instructions. Compute sample ph at important stages of a titration. Calculate the. Acid Base Titration Of Hcl And Naoh.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Acid Base Titration Of Hcl And Naoh Compute sample ph at important stages of a titration. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Adding naoh decreases the concentration of h + because of the neutralization. Includes kit list and safety instructions. Use this class practical to explore titration, producing. Acid Base Titration Of Hcl And Naoh.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Compute sample ph at important stages of a titration. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Includes kit list and safety instructions. Calculate the. Acid Base Titration Of Hcl And Naoh.
From www.youtube.com
Titration of a monoprotic strong acid (HCl) and monoprotic strong base Acid Base Titration Of Hcl And Naoh Includes kit list and safety instructions. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Compute sample ph at important stages of a titration. Calculate the. Acid Base Titration Of Hcl And Naoh.
From www.slideserve.com
PPT Acidbase titration PowerPoint Presentation, free download ID Acid Base Titration Of Hcl And Naoh Compute sample ph at important stages of a titration. Includes kit list and safety instructions. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. Adding. Acid Base Titration Of Hcl And Naoh.
From studylib.net
Titration of HCl with NaOH Acid Base Titration Of Hcl And Naoh Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at the listed. Compute sample ph at important stages of a titration. A titration is a technique, in which a reagent, called a titrant, of known concentration. Use this class practical to explore titration, producing the salt. Acid Base Titration Of Hcl And Naoh.
From www.numerade.com
Lab Titration of HCl and NaOH to Determine the Concentration of NaOH Acid Base Titration Of Hcl And Naoh Use this class practical to explore titration, producing the salt sodium chloride with sodium hydroxide and hydrochloric acid. A titration is a technique, in which a reagent, called a titrant, of known concentration. Because hcl is a strong acid that is completely ionized in water, the initial [h +] is 0.10 m, and the initial ph is 1.00. Compute sample. Acid Base Titration Of Hcl And Naoh.