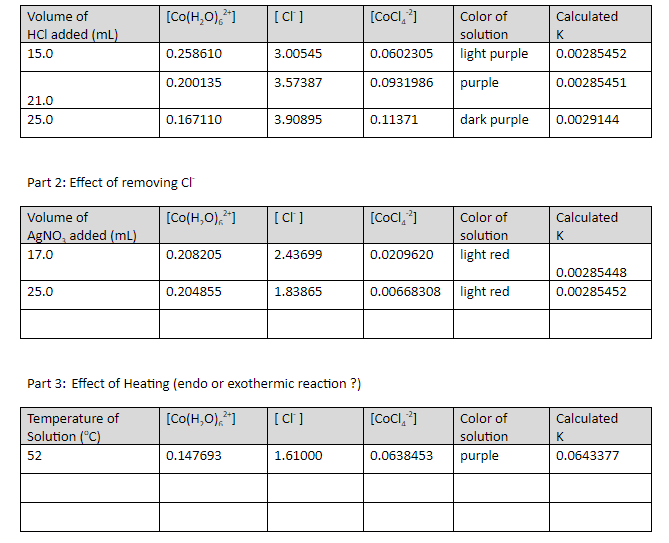
Le Chatelier's Principle Virtual Lab Answers . Marking stress → 1 mark observation → 1 mark explanation → 3 marks. 𝐹𝑒 (aq) + (aq) (aq) + heat: Click the card to flip 👆. Le chatelier's principle practice interactive. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Select the equilibrium section under virtual labs. You can do this activity either online or offline. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. If a system at equilibrium is affected by. Cobalt system (/25) chemical equation:.
from www.chegg.com
Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Cobalt system (/25) chemical equation:. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. 𝐹𝑒 (aq) + (aq) (aq) + heat: Le chatelier's principle practice interactive. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. Click the card to flip 👆. You can do this activity either online or offline. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these.
Solved CHEMICAL EQUILIBRIUM & Le CHATELIER’S
Le Chatelier's Principle Virtual Lab Answers Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. You can do this activity either online or offline. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. 𝐹𝑒 (aq) + (aq) (aq) + heat: Cobalt system (/25) chemical equation:. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. If a system at equilibrium is affected by. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Le chatelier's principle practice interactive. Click the card to flip 👆. Select the equilibrium section under virtual labs. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in.
From morioh.com
Le Chatelier's Principle Le Chatelier's Principle Virtual Lab Answers Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Select the equilibrium section under virtual labs. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. Le chatelier's principle practice interactive. Le chatelier's principle states that if a stress is applied to. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
CHEMICAL EQUILIBRIUM& Le CHATELIER'S PRINCIPLE Le Chatelier's Principle Virtual Lab Answers Click the card to flip 👆. Select the equilibrium section under virtual labs. 𝐹𝑒 (aq) + (aq) (aq) + heat: The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. If a system at equilibrium is affected by. Marking stress → 1 mark observation → 1 mark explanation → 3. Le Chatelier's Principle Virtual Lab Answers.
From worksheetlibehrlich.z19.web.core.windows.net
Le Chatelier's Principle Worksheet Answer Key Le Chatelier's Principle Virtual Lab Answers 𝐹𝑒 (aq) + (aq) (aq) + heat: Cobalt system (/25) chemical equation:. Le chatelier's principle practice interactive. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. Select the equilibrium section under. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved Virtual Lab Le Chatelier's Principle Inquiry Le Chatelier's Principle Virtual Lab Answers Click the card to flip 👆. If a system at equilibrium is affected by. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Le chatelier's principle practice interactive. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to. Le Chatelier's Principle Virtual Lab Answers.
From oneclass.com
OneClass LAB REPORT Prelab questions 1. Describe a nonchemical Le Chatelier's Principle Virtual Lab Answers The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. If a system at equilibrium is affected by. You can do this activity either online or offline. Cobalt system (/25) chemical equation:. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Le chatelier's principle practice interactive.. Le Chatelier's Principle Virtual Lab Answers.
From www.artofit.org
Le chatelier s principle Artofit Le Chatelier's Principle Virtual Lab Answers Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. You can do this activity either online or offline. Click. Le Chatelier's Principle Virtual Lab Answers.
From studylib.net
Le Chatelier`s Principle Online Lab Le Chatelier's Principle Virtual Lab Answers Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Cobalt system (/25) chemical equation:. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Select the equilibrium section under virtual labs. The objectives of this experiment are to perturb chemical reactions at equilibrium. Le Chatelier's Principle Virtual Lab Answers.
From www.studocu.com
Virtual lab lechatlier CHEMICAL EQUILIBRIUM Le PRINCIPLE Virtual Lab Le Chatelier's Principle Virtual Lab Answers If a system at equilibrium is affected by. Cobalt system (/25) chemical equation:. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side.. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved 4. Chemical Equilibrium and Le Chatelier's Principle Le Chatelier's Principle Virtual Lab Answers Marking stress → 1 mark observation → 1 mark explanation → 3 marks. If a system at equilibrium is affected by. 𝐹𝑒 (aq) + (aq) (aq) + heat: The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. You can do this activity either online or offline. Cobalt system (/25). Le Chatelier's Principle Virtual Lab Answers.
From www.studypool.com
SOLUTION Le chatelier s princible virtual lab Studypool Le Chatelier's Principle Virtual Lab Answers The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. Select the equilibrium section under virtual labs. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Marking stress → 1 mark observation → 1 mark explanation. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved Chemical Equilibrium Le Chatelier’s Principle Lab. I Le Chatelier's Principle Virtual Lab Answers Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. If a system at equilibrium is affected by. Le chatelier's principle practice interactive. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. Click the card to flip 👆. The objectives of this. Le Chatelier's Principle Virtual Lab Answers.
From www.studypool.com
SOLUTION Le chatelier s princible virtual lab Studypool Le Chatelier's Principle Virtual Lab Answers Cobalt system (/25) chemical equation:. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. If a system at equilibrium is affected by. You can do this activity either online or offline. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction. Le Chatelier's Principle Virtual Lab Answers.
From www.studocu.com
Project 4 Le Chatelier's Principle 3 ChemCollective Virtual Lab Le Chatelier's Principle Virtual Lab Answers Select the equilibrium section under virtual labs. If a system at equilibrium is affected by. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. Click the card to flip 👆. Le chatelier's principle practice interactive. Cobalt system (/25) chemical equation:. The objectives of this experiment. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved Virtual Lab Le Chatelier's Principle Inquiry Le Chatelier's Principle Virtual Lab Answers Select the equilibrium section under virtual labs. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Le chatelier's principle practice interactive. Click the card to flip 👆. Cobalt chloride and lechatlier’s principle prepared. Le Chatelier's Principle Virtual Lab Answers.
From www.chemistrylearner.com
Free Printable Le Chatelier's Principle Worksheets Le Chatelier's Principle Virtual Lab Answers Select the equilibrium section under virtual labs. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. You can do this activity either online or offline. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Le. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved Chemical Equilibrium Le Chatelier’s Principle Lab. I Le Chatelier's Principle Virtual Lab Answers If a system at equilibrium is affected by. You can do this activity either online or offline. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. Cobalt system (/25) chemical equation:. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. 𝐹𝑒 (aq) + (aq) (aq). Le Chatelier's Principle Virtual Lab Answers.
From imsucc.blogspot.com
Le Chateliers Principle Virtual Lab Answers Get Images Le Chatelier's Principle Virtual Lab Answers Le chatelier's principle practice interactive. If a system at equilibrium is affected by. Cobalt system (/25) chemical equation:. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. Click the card to flip 👆. Cobalt chloride and. Le Chatelier's Principle Virtual Lab Answers.
From socratic.org
What is Le Chatelier's principle in chemistry? Socratic Le Chatelier's Principle Virtual Lab Answers Click the card to flip 👆. Select the equilibrium section under virtual labs. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Le chatelier's principle states that if a stress is applied to a reversible reaction. Le Chatelier's Principle Virtual Lab Answers.
From www.studypool.com
SOLUTION Le Chateliers Principle Lab Simulation Equilibrium Solved Le Chatelier's Principle Virtual Lab Answers 𝐹𝑒 (aq) + (aq) (aq) + heat: Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. You can do this activity either online or offline. Select the equilibrium section under virtual labs. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic. Le Chatelier's Principle Virtual Lab Answers.
From studylib.net
Chem project_012112_pre and post lab questions_edit_answers Le Chatelier's Principle Virtual Lab Answers Cobalt system (/25) chemical equation:. You can do this activity either online or offline. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. The objectives of this experiment are to perturb chemical. Le Chatelier's Principle Virtual Lab Answers.
From desmond-has-mayer.blogspot.com
Use Le Chatelier's Principle to Explain the Different Colors Desmond Le Chatelier's Principle Virtual Lab Answers Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Cobalt system (/25) chemical equation:. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. Click the card to flip 👆. 𝐹𝑒 (aq) + (aq) (aq) + heat: Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction. Le Chatelier's Principle Virtual Lab Answers.
From www.studocu.com
Le chatelier online lab pre lab answers printable Le Chatelier’s Le Chatelier's Principle Virtual Lab Answers Select the equilibrium section under virtual labs. Cobalt system (/25) chemical equation:. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. If a system at equilibrium is affected by. Le chatelier's principle practice interactive. Click the card to flip 👆. You can do this activity either online or offline. Le chatelier's principle states that if. Le Chatelier's Principle Virtual Lab Answers.
From www.youtube.com
How to do Lab Report 005 Le Chatelier's Principle YouTube Le Chatelier's Principle Virtual Lab Answers If a system at equilibrium is affected by. 𝐹𝑒 (aq) + (aq) (aq) + heat: The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Select the equilibrium section under virtual labs. Cobalt system (/25) chemical equation:.. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved Chemical Equilibrium Le Chatelier’s Principle Lab. I Le Chatelier's Principle Virtual Lab Answers You can do this activity either online or offline. Cobalt system (/25) chemical equation:. 𝐹𝑒 (aq) + (aq) (aq) + heat: If a system at equilibrium is affected by. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. Click the card to flip 👆. Cobalt. Le Chatelier's Principle Virtual Lab Answers.
From www.docsity.com
Le Châtelier’s Principle Lab Report Lab Reports Chemistry Docsity Le Chatelier's Principle Virtual Lab Answers Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. Le chatelier's principle practice interactive. Select the equilibrium section under virtual labs. 𝐹𝑒 (aq) + (aq) (aq) + heat: Marking stress → 1 mark observation → 1 mark explanation → 3 marks. You can do this. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved Prelab Questions Le Châtelier's Principle Before Le Chatelier's Principle Virtual Lab Answers Click the card to flip 👆. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Select the equilibrium section under virtual labs. If a system at equilibrium is affected by. Marking stress. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved EXPERIMENT 73 Le Châtelier's Principle Le Chatelier's Principle Virtual Lab Answers Marking stress → 1 mark observation → 1 mark explanation → 3 marks. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. Click the card to flip 👆. Select the equilibrium section under virtual labs. You can do this activity either online or offline. The. Le Chatelier's Principle Virtual Lab Answers.
From worksheetcampuschiba.z21.web.core.windows.net
Worksheet Le Chatelier's Principle Le Chatelier's Principle Virtual Lab Answers Select the equilibrium section under virtual labs. Cobalt system (/25) chemical equation:. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. If a system at equilibrium is affected by. Le chatelier's principle states that if a. Le Chatelier's Principle Virtual Lab Answers.
From oneclass.com
OneClass Any help/explanations with this Le Chatelier's Principle lab Le Chatelier's Principle Virtual Lab Answers Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link. Le Chatelier's Principle Virtual Lab Answers.
From imsucc.blogspot.com
Le Chateliers Principle Lab Answers Get Images Le Chatelier's Principle Virtual Lab Answers Select the equilibrium section under virtual labs. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. Cobalt system (/25) chemical equation:. Le chatelier's principle practice interactive. You can do this activity either online or offline. If a system at equilibrium is affected by. Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved Date Le Chatelier's Principle Prelab 1. Define Le Le Chatelier's Principle Virtual Lab Answers 𝐹𝑒 (aq) + (aq) (aq) + heat: Cobalt system (/25) chemical equation:. Marking stress → 1 mark observation → 1 mark explanation → 3 marks. If a system at equilibrium is affected by. You can do this activity either online or offline. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to. Le Chatelier's Principle Virtual Lab Answers.
From www.studypool.com
SOLUTION Le Chateliers Principle Lab Simulation Equilibrium Solved Le Chatelier's Principle Virtual Lab Answers You can do this activity either online or offline. Cobalt system (/25) chemical equation:. Select the equilibrium section under virtual labs. Click the card to flip 👆. The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. Le chatelier's principle practice interactive. If a system at equilibrium is affected by.. Le Chatelier's Principle Virtual Lab Answers.
From www.studypool.com
SOLUTION Le chatelier s princible virtual lab Studypool Le Chatelier's Principle Virtual Lab Answers Click the card to flip 👆. Cobalt system (/25) chemical equation:. If a system at equilibrium is affected by. Le chatelier's principle states that if a stress is applied to a reversible reaction at equilibrium, the reaction will undergo a shift in. Select the equilibrium section under virtual labs. 𝐹𝑒 (aq) + (aq) (aq) + heat: You can do this. Le Chatelier's Principle Virtual Lab Answers.
From gallerycarla15.blogspot.com
Worksheet Le Chateliers Principle Equilibrium And Lechatelier S Le Chatelier's Principle Virtual Lab Answers The objectives of this experiment are to perturb chemical reactions at equilibrium and observe how they respond, to explain these. Click the card to flip 👆. 𝐹𝑒 (aq) + (aq) (aq) + heat: Le châtelier’s principle shows that this is an 3+ 𝑆𝐶𝑁 − 𝐹𝑒𝑆𝐶𝑁 2+ exothermic reaction since heat is being released on the product side. Cobalt chloride and. Le Chatelier's Principle Virtual Lab Answers.
From www.chegg.com
Solved CHEMICAL EQUILIBRIUM & Le CHATELIER’S Le Chatelier's Principle Virtual Lab Answers Click the card to flip 👆. Le chatelier's principle practice interactive. Cobalt chloride and lechatlier’s principle prepared by sathya perera go to the link chemcollective/vlabs. Cobalt system (/25) chemical equation:. If a system at equilibrium is affected by. You can do this activity either online or offline. Marking stress → 1 mark observation → 1 mark explanation → 3 marks.. Le Chatelier's Principle Virtual Lab Answers.