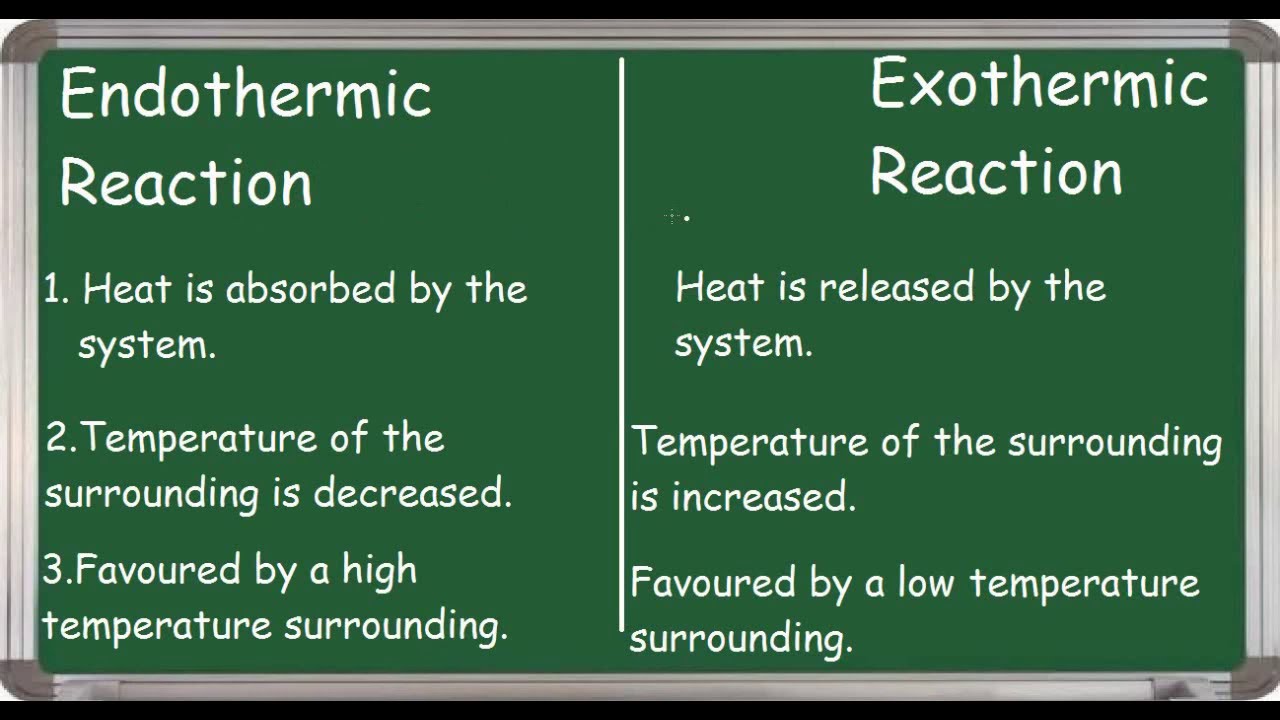
Why Is Boiling Water Endothermic . In the course of an. Boiling is an endothermic process because it requires energy input to overcome the. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction feels cold because it absorbs heat from its surroundings. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling water is an endothermic process. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. This is because energy is required. This means it absorbs heat from its surroundings. Why is boiling an endothermic process?
from klajlbzfp.blob.core.windows.net
Why is boiling an endothermic process? This is because energy is required. Boiling water is an endothermic process. An endothermic reaction feels cold because it absorbs heat from its surroundings. In the course of an. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This means it absorbs heat from its surroundings. Boiling is an endothermic process because it requires energy input to overcome the. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy.
Endothermic Solution Examples at Jeffrey Pleasants blog
Why Is Boiling Water Endothermic Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Why is boiling an endothermic process? Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. This means it absorbs heat from its surroundings. Boiling water is an endothermic process. Boiling is an endothermic process because it requires energy input to overcome the. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction feels cold because it absorbs heat from its surroundings. In the course of an. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. This is because energy is required.
From cartoondealer.com
Electrolysis Cartoons, Illustrations & Vector Stock Images 60 Why Is Boiling Water Endothermic Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. An endothermic reaction feels cold because it absorbs heat from its surroundings. This means it absorbs heat from its surroundings. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Why is boiling an endothermic process? A chemical reaction or. Why Is Boiling Water Endothermic.
From slideplayer.com
CHAPTER 10 ENERGY. ppt download Why Is Boiling Water Endothermic Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. This means it absorbs heat from its surroundings. An endothermic reaction feels cold because it absorbs heat from its surroundings. Boiling water is an endothermic process. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal. Why Is Boiling Water Endothermic.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Why Is Boiling Water Endothermic Why is boiling an endothermic process? An endothermic reaction feels cold because it absorbs heat from its surroundings. In the course of an. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. This means it absorbs heat from its. Why Is Boiling Water Endothermic.
From alisonnewspatterson.blogspot.com
Photosynthesis is Exothermic or Endothermic Why Is Boiling Water Endothermic A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This is because energy is required. In the course of an. Boiling water is an endothermic process. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. An endothermic reaction feels cold because it absorbs. Why Is Boiling Water Endothermic.
From www.youtube.com
Is boiling water endothermic or exothermic with respect to water? YouTube Why Is Boiling Water Endothermic An endothermic reaction feels cold because it absorbs heat from its surroundings. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Boiling water is an endothermic process. This means it absorbs heat from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the. Why Is Boiling Water Endothermic.
From www.numerade.com
SOLVED Be sure to answer all parts Classify each process as Why Is Boiling Water Endothermic Why is boiling an endothermic process? Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. An endothermic reaction feels cold because it absorbs heat from its surroundings. Boiling water is an endothermic process. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Boiling is an endothermic process because. Why Is Boiling Water Endothermic.
From nathanaelsrharding.blogspot.com
Is Combustion Endothermic or Exothermic NathanaelsrHarding Why Is Boiling Water Endothermic A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This means it absorbs heat from its surroundings. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Boiling water is when. Why Is Boiling Water Endothermic.
From exykqwqcv.blob.core.windows.net
Describe Process Of Boiling Water For Drinking at Timothy Speciale blog Why Is Boiling Water Endothermic A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This is because energy is required. This means it absorbs heat from its surroundings. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and. Why Is Boiling Water Endothermic.
From www.youtube.com
What causes Severe Burns Steam or Boiling water ? YouTube Why Is Boiling Water Endothermic Boiling is an endothermic process because it requires energy input to overcome the. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Examples of endothermic reactions include photosynthesis, dissolving salt. Why Is Boiling Water Endothermic.
From stock.adobe.com
Endothermic reactions list with external energy outline collection set Why Is Boiling Water Endothermic This is because energy is required. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Boiling is an endothermic process because it requires energy input to overcome the. In the course of an. Why is boiling an endothermic process? Boiling water is when water changes phase from a liquid to a gas (water vapor) due to. Why Is Boiling Water Endothermic.
From www.baamboozle.com
Energy Change and Reaction Rate Baamboozle Baamboozle The Most Why Is Boiling Water Endothermic An endothermic reaction feels cold because it absorbs heat from its surroundings. This is because energy is required. Boiling water is an endothermic process. Boiling is an endothermic process because it requires energy input to overcome the. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an.. Why Is Boiling Water Endothermic.
From www.teachoo.com
Which of the reactions is an endothermic reaction? MCQ Science Why Is Boiling Water Endothermic A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Why is boiling an endothermic process? In the course of an. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. An endothermic reaction feels cold because it absorbs heat. Why Is Boiling Water Endothermic.
From www.thoughtco.com
Endothermic Reaction Examples Why Is Boiling Water Endothermic Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. Why is boiling an endothermic process? This means it absorbs heat from its surroundings. In the course of an. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. A chemical reaction or physical change is endothermic if heat is. Why Is Boiling Water Endothermic.
From www.slideserve.com
PPT Exothermic and Endothermic Processes PowerPoint Presentation Why Is Boiling Water Endothermic This is because energy is required. Boiling is an endothermic process because it requires energy input to overcome the. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. In the course of an. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This means it absorbs heat. Why Is Boiling Water Endothermic.
From www.slideserve.com
PPT Endothermic Reaction Investigation Ammonium Chloride + Water Why Is Boiling Water Endothermic In the course of an. Why is boiling an endothermic process? This means it absorbs heat from its surroundings. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. An endothermic reaction feels cold because it absorbs heat from its surroundings. Boiling water is an endothermic process. Examples of endothermic reactions include photosynthesis,. Why Is Boiling Water Endothermic.
From tapwaterfeatures.com
Is Boiling Water Endothermic or Exothermic Why Is Boiling Water Endothermic Boiling is an endothermic process because it requires energy input to overcome the. An endothermic reaction feels cold because it absorbs heat from its surroundings. This is because energy is required. In the course of an. Boiling water is an endothermic process. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Boiling water is an endothermic. Why Is Boiling Water Endothermic.
From tapwaterfeatures.com
Is Boiling Water Endothermic or Exothermic Why Is Boiling Water Endothermic A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction feels cold because it absorbs heat from its surroundings. This means it absorbs heat from its surroundings. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy.. Why Is Boiling Water Endothermic.
From klajtmkuv.blob.core.windows.net
Is Boiling Water A Spontaneous Process at Andre Hefner blog Why Is Boiling Water Endothermic Boiling is an endothermic process because it requires energy input to overcome the. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction feels cold because it absorbs heat from its surroundings. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy.. Why Is Boiling Water Endothermic.
From www.slideserve.com
PPT DESCRIBE PHYSICAL & CHEMICAL CHANGES IN TERMS OF ENDOTHERMIC Why Is Boiling Water Endothermic An endothermic reaction feels cold because it absorbs heat from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This means it absorbs heat from its surroundings. This is because energy is required. Boiling is an endothermic process because it requires energy input to overcome the. Boiling water is. Why Is Boiling Water Endothermic.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Why Is Boiling Water Endothermic Why is boiling an endothermic process? An endothermic reaction feels cold because it absorbs heat from its surroundings. Boiling is an endothermic process because it requires energy input to overcome the. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling water is an endothermic process. Boiling. Why Is Boiling Water Endothermic.
From mudfooted.com
Endothermic Animals The Benefits Of Generating Your Own Heat MudFooted Why Is Boiling Water Endothermic Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. This is because energy is required. Boiling water is an endothermic process. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. A chemical reaction or physical change is endothermic if. Why Is Boiling Water Endothermic.
From www.teachoo.com
What produces more severe burns, boiling water or steam? (Teachoo) Why Is Boiling Water Endothermic An endothermic reaction feels cold because it absorbs heat from its surroundings. Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling is an endothermic process because it requires energy input to overcome the. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold.. Why Is Boiling Water Endothermic.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Why Is Boiling Water Endothermic A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This is because energy is required. Why is boiling an endothermic process? Boiling water is an endothermic process. Boiling is an endothermic process because it requires energy input to overcome the. This means it absorbs heat from its surroundings. Examples of endothermic. Why Is Boiling Water Endothermic.
From ask.modifiyegaraj.com
Difference Between Endothermic And Exothermic Reactions Chemistry Why Is Boiling Water Endothermic Boiling water is an endothermic process. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. This means it absorbs heat from its surroundings. An endothermic reaction feels cold because it absorbs heat from its surroundings. Why is boiling an endothermic process? A chemical reaction or physical change is endothermic if heat is absorbed by the system. Why Is Boiling Water Endothermic.
From www.gauthmath.com
Solved Why is boiling water a physical change? A. It is a state of Why Is Boiling Water Endothermic Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. This means it absorbs heat from its surroundings. Boiling is an endothermic process because it requires energy input to overcome the. An endothermic reaction feels cold because it absorbs heat from its surroundings. In the course of an. Boiling water is an endothermic process, which supplies heat. Why Is Boiling Water Endothermic.
From ar.inspiredpencil.com
Water Chemical Equation Why Is Boiling Water Endothermic This means it absorbs heat from its surroundings. This is because energy is required. An endothermic reaction feels cold because it absorbs heat from its surroundings. Boiling water is an endothermic process. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the course of an. Why is boiling an endothermic. Why Is Boiling Water Endothermic.
From calvinkruwpace.blogspot.com
Is Photosynthesis Endothermic or Exothermic CalvinkruwPace Why Is Boiling Water Endothermic This is because energy is required. Boiling is an endothermic process because it requires energy input to overcome the. An endothermic reaction feels cold because it absorbs heat from its surroundings. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Boiling water is an endothermic process, which supplies heat to the. Why Is Boiling Water Endothermic.
From socratic.org
Why is vaporization endothermic? Why is condensation exothermic? Socratic Why Is Boiling Water Endothermic Boiling is an endothermic process because it requires energy input to overcome the. This means it absorbs heat from its surroundings. In the course of an. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This is because energy is required. Boiling water is an endothermic process. Boiling water is an. Why Is Boiling Water Endothermic.
From klaoasptt.blob.core.windows.net
Does Boiling Water Leave Scars at Diane Pierre blog Why Is Boiling Water Endothermic Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Boiling is an endothermic process because it requires energy input to overcome the. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical. Why Is Boiling Water Endothermic.
From www.diffzy.com
Evaporation vs Boiling What's the Difference (With Table) Why Is Boiling Water Endothermic This means it absorbs heat from its surroundings. This is because energy is required. Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. An endothermic reaction feels cold because it absorbs heat from its. Why Is Boiling Water Endothermic.
From tapwaterfeatures.com
Is Boiling Water Endothermic or Exothermic Why Is Boiling Water Endothermic In the course of an. This means it absorbs heat from its surroundings. This is because energy is required. An endothermic reaction feels cold because it absorbs heat from its surroundings. Why is boiling an endothermic process? Boiling water is an endothermic process. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. Boiling is an endothermic. Why Is Boiling Water Endothermic.
From exobyexop.blob.core.windows.net
Is Butane Gas Burning In A Lighter Exothermic Or Endothermic at Chanel Why Is Boiling Water Endothermic Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. In the course of an. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This means it absorbs heat from its surroundings. Boiling water is an endothermic process. Boiling. Why Is Boiling Water Endothermic.
From www.numerade.com
SOLVED Which of these is the TRUE statement? A) Combustion reactions Why Is Boiling Water Endothermic Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Why is boiling an endothermic process? Boiling water is an endothermic process, which supplies heat to the water molecules, increasing their potential energy. A chemical reaction or physical change is endothermic if heat is absorbed by the system. Why Is Boiling Water Endothermic.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Why Is Boiling Water Endothermic Boiling water is an endothermic process. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. This is because energy is required. Boiling is an endothermic process because it requires energy input to overcome the. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold. An endothermic reaction feels. Why Is Boiling Water Endothermic.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Why Is Boiling Water Endothermic Boiling water is when water changes phase from a liquid to a gas (water vapor) due to the absorption of thermal energy. Boiling is an endothermic process because it requires energy input to overcome the. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Examples of endothermic reactions include photosynthesis, dissolving. Why Is Boiling Water Endothermic.