Terminal Alkenes Examples . For organic compounds, a conventional way to tell. alkenes undergo a number of reactions in which the c=c double bond is oxidized. illustrated glossary of organic chemistry. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of.
from thechemistrynotes.com
alkenes undergo a number of reactions in which the c=c double bond is oxidized. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. illustrated glossary of organic chemistry. For organic compounds, a conventional way to tell. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with.
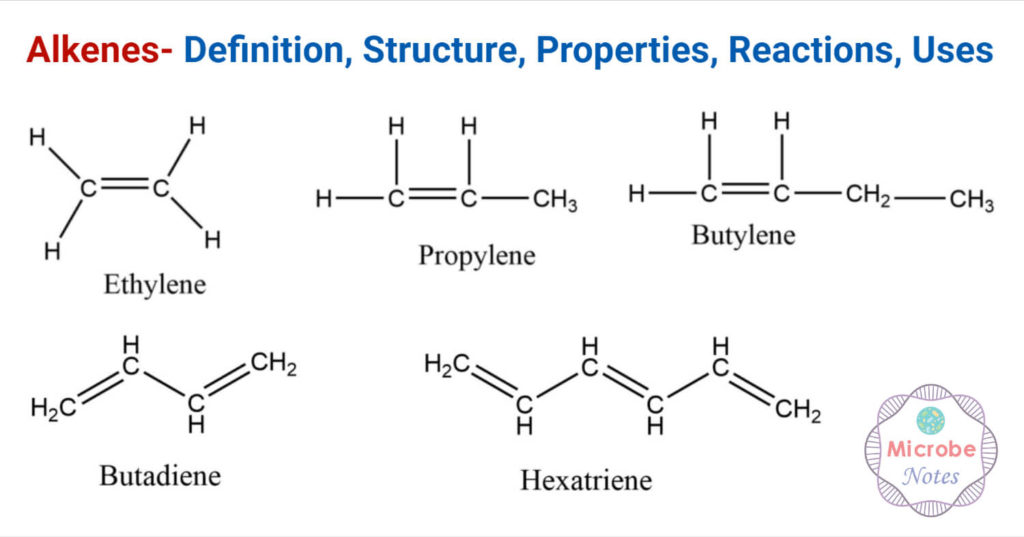
Alkenes Definition, Structure, Properties, Reactions, Uses
Terminal Alkenes Examples alkenes undergo a number of reactions in which the c=c double bond is oxidized. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. illustrated glossary of organic chemistry. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. For organic compounds, a conventional way to tell. alkenes undergo a number of reactions in which the c=c double bond is oxidized. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6.
From pdfprof.com
reactions of alkenes summary Terminal Alkenes Examples alkenes undergo a number of reactions in which the c=c double bond is oxidized. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. such examples are found in transformations of industrial. Terminal Alkenes Examples.
From www.chemistrysteps.com
Alkenes Structure and Stability Chemistry Steps Terminal Alkenes Examples alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. illustrated glossary of organic chemistry. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. alkenes undergo a number of reactions in which the. Terminal Alkenes Examples.
From in.pinterest.com
Internal alkene to terminal alkene. Chemistry, Organic chemistry Terminal Alkenes Examples this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. alkenes. Terminal Alkenes Examples.
From owlcation.com
The Chemistry of Alkenes Structure, Naming, Uses & Reactions Owlcation Terminal Alkenes Examples although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. illustrated glossary of organic chemistry. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. alkenes undergo a number of reactions in which the c=c double bond. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Alkene Nomenclature Cis and Trans and E and Z — Master Organic Chemistry Terminal Alkenes Examples alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. For organic compounds, a conventional way to tell. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. this system enables the transformation of terminal. Terminal Alkenes Examples.
From www.chemistrysteps.com
Naming Alkenes Chemistry Steps Terminal Alkenes Examples although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. For organic compounds, a conventional way to tell. illustrated glossary of organic chemistry. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. alkenes undergo a number. Terminal Alkenes Examples.
From chemistryguru.com.sg
Oxidative Cleavage of Alkenes Terminal Alkenes Examples although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. illustrated glossary of organic chemistry. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. alkenes are molecules with carbons bonded to hydrogens which contain at. Terminal Alkenes Examples.
From www.aceorganicchem.com
Alkene Reactions [with useful chart] Organic chemistry help by Terminal Alkenes Examples although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. alkenes undergo a number of reactions in which the c=c double bond is oxidized. illustrated glossary of organic chemistry. this system enables the transformation of terminal alkynes, even in the presence of. Terminal Alkenes Examples.
From www.chemtube3d.com
Electrophilic addition to alkenes is stereospecific Terminal Alkenes Examples this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. For organic compounds, a conventional way to tell. illustrated glossary of organic chemistry. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. alkenes undergo a number of reactions in which the c=c double bond. Terminal Alkenes Examples.
From www.slideserve.com
PPT Reactions of Alkenes PowerPoint Presentation, free download ID Terminal Alkenes Examples this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. alkenes undergo a number of reactions in which the c=c double bond is oxidized. For organic compounds, a conventional way to tell. illustrated glossary of. Terminal Alkenes Examples.
From www.researchgate.net
A) Examples of terminal alkenecontaining polyketide natural products Terminal Alkenes Examples For organic compounds, a conventional way to tell. alkenes undergo a number of reactions in which the c=c double bond is oxidized. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. illustrated glossary of. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Summary Three Key Families Of Alkene Reaction Mechanisms Terminal Alkenes Examples although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. For organic compounds, a conventional way to tell. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. such examples are found in transformations of. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Summary Three Key Families Of Alkene Reaction Mechanisms Terminal Alkenes Examples illustrated glossary of organic chemistry. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. For organic compounds, a conventional way to tell. alkenes undergo a number of reactions in which the c=c double bond is oxidized. alkenes are molecules with carbons. Terminal Alkenes Examples.
From chemistnotes.com
Alkenes formula, structure, nomenclature, properties, and uses Terminal Alkenes Examples For organic compounds, a conventional way to tell. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. illustrated glossary of organic chemistry. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. alkenes are molecules with. Terminal Alkenes Examples.
From thechemistrynotes.com
Alkenes Definition, Structure, Properties, Reactions, Uses Terminal Alkenes Examples illustrated glossary of organic chemistry. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. alkenes undergo a number of reactions in which the c=c double bond is oxidized. For organic compounds, a conventional way to tell. although there is only one alkene with the formula c 2 h 4. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Synthesis (4) Alkene Reaction Map, Including Alkyl Halide Reactions Terminal Alkenes Examples alkenes undergo a number of reactions in which the c=c double bond is oxidized. For organic compounds, a conventional way to tell. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. illustrated glossary of organic chemistry. alkenes are molecules with carbons bonded to hydrogens which contain at least two. Terminal Alkenes Examples.
From chemistryguru.com.sg
Strong Oxidation of Alkene Worked Example Terminal Alkenes Examples alkenes undergo a number of reactions in which the c=c double bond is oxidized. For organic compounds, a conventional way to tell. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. illustrated glossary of organic chemistry. such examples are found in transformations of industrial importance, such as the terminal. Terminal Alkenes Examples.
From medium.com
An Overview of Alkene Chemistry. Alkenes Structure & Reactivity by Terminal Alkenes Examples such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. For organic compounds, a conventional way to tell. alkenes are molecules with carbons bonded to hydrogens which contain. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Acid Catalyzed Hydration of Alkenes Follows A Key Mechanistic Pattern Terminal Alkenes Examples alkenes undergo a number of reactions in which the c=c double bond is oxidized. illustrated glossary of organic chemistry. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. alkenes are molecules with carbons bonded to hydrogens which contain at least two. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Synthesis (5) Reactions of Alkynes Master Organic Chemistry Terminal Alkenes Examples although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. alkenes undergo a number of reactions in which the c=c double bond is oxidized. For organic compounds, a conventional way to tell. such examples are found in transformations of industrial importance, such as. Terminal Alkenes Examples.
From pdfprof.com
halogénation des alcanes Terminal Alkenes Examples alkenes undergo a number of reactions in which the c=c double bond is oxidized. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. illustrated glossary of organic chemistry. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Alkyne Reaction Patterns The Carbocation Pathway — Master Organic Terminal Alkenes Examples illustrated glossary of organic chemistry. alkenes undergo a number of reactions in which the c=c double bond is oxidized. For organic compounds, a conventional way to tell. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. although there is only one alkene with the formula c 2 h 4. Terminal Alkenes Examples.
From mungfali.com
Alkene Structural Formula Terminal Alkenes Examples For organic compounds, a conventional way to tell. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with.. Terminal Alkenes Examples.
From www.slideserve.com
PPT Alkanes Structure and Conformation PowerPoint Presentation, free Terminal Alkenes Examples although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. alkenes undergo a number of reactions in which the c=c double bond is oxidized. For organic compounds, a conventional way to tell. such examples are found in transformations of industrial importance, such as. Terminal Alkenes Examples.
From www.slideserve.com
PPT Alkanes Structure and Conformation PowerPoint Presentation, free Terminal Alkenes Examples illustrated glossary of organic chemistry. For organic compounds, a conventional way to tell. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. although there is only one alkene with the formula. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Alkynes and Synthesis Master Organic Chemistry Terminal Alkenes Examples illustrated glossary of organic chemistry. For organic compounds, a conventional way to tell. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. such examples are. Terminal Alkenes Examples.
From chemistnotes.com
Alkenes formula, structure, nomenclature, properties, and uses Terminal Alkenes Examples illustrated glossary of organic chemistry. alkenes undergo a number of reactions in which the c=c double bond is oxidized. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. For organic compounds, a conventional way to tell. this system enables the transformation of terminal alkynes, even in the. Terminal Alkenes Examples.
From englelab.com
Stereodivergent NickelCatalyzed Isomerization of Terminal Alkenes Terminal Alkenes Examples although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. For organic compounds, a conventional way to tell. illustrated glossary of organic chemistry. alkenes are molecules. Terminal Alkenes Examples.
From www.chemistrysteps.com
Ozonolysis of Alkenes with Practice Problems Chemistry Steps Terminal Alkenes Examples this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. illustrated glossary of organic chemistry. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. For organic compounds, a conventional way to tell. alkenes undergo a. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Alkyne Addition Reactions The “Concerted” Pathway — Master Organic Terminal Alkenes Examples illustrated glossary of organic chemistry. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. alkenes undergo a number of reactions in which the c=c double. Terminal Alkenes Examples.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes — Master Organic Chemistry Terminal Alkenes Examples although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. alkenes undergo a number of reactions in which the c=c double bond is oxidized. For organic compounds, a conventional way to tell. illustrated glossary of organic chemistry. this system enables the transformation. Terminal Alkenes Examples.
From chemistnotes.com
Ozonolysis of alkenes and alkynes Mechanism, examples Chemistry Notes Terminal Alkenes Examples alkenes undergo a number of reactions in which the c=c double bond is oxidized. illustrated glossary of organic chemistry. such examples are found in transformations of industrial importance, such as the terminal hydroformylation of. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. although there is only one. Terminal Alkenes Examples.
From fity.club
Naming Alkenes By Iupac Nomenclature Rules With Images Terminal Alkenes Examples illustrated glossary of organic chemistry. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. such examples are found in transformations of industrial importance, such as. Terminal Alkenes Examples.
From studylib.net
Chapter O 10 Terminal Alkenes Examples this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. although there is only one alkene with the formula c 2 h 4 (ethene) and only one with the formula c 3 h 6. For organic compounds, a conventional way to tell. alkenes undergo a number of reactions in which the. Terminal Alkenes Examples.
From www.pinterest.co.kr
Alkyne reactions summary practice problems Organic chemistry Terminal Alkenes Examples For organic compounds, a conventional way to tell. this system enables the transformation of terminal alkynes, even in the presence of bulky substituents, with. alkenes are molecules with carbons bonded to hydrogens which contain at least two sp 2 hybridized carbon atoms. illustrated glossary of organic chemistry. such examples are found in transformations of industrial importance,. Terminal Alkenes Examples.