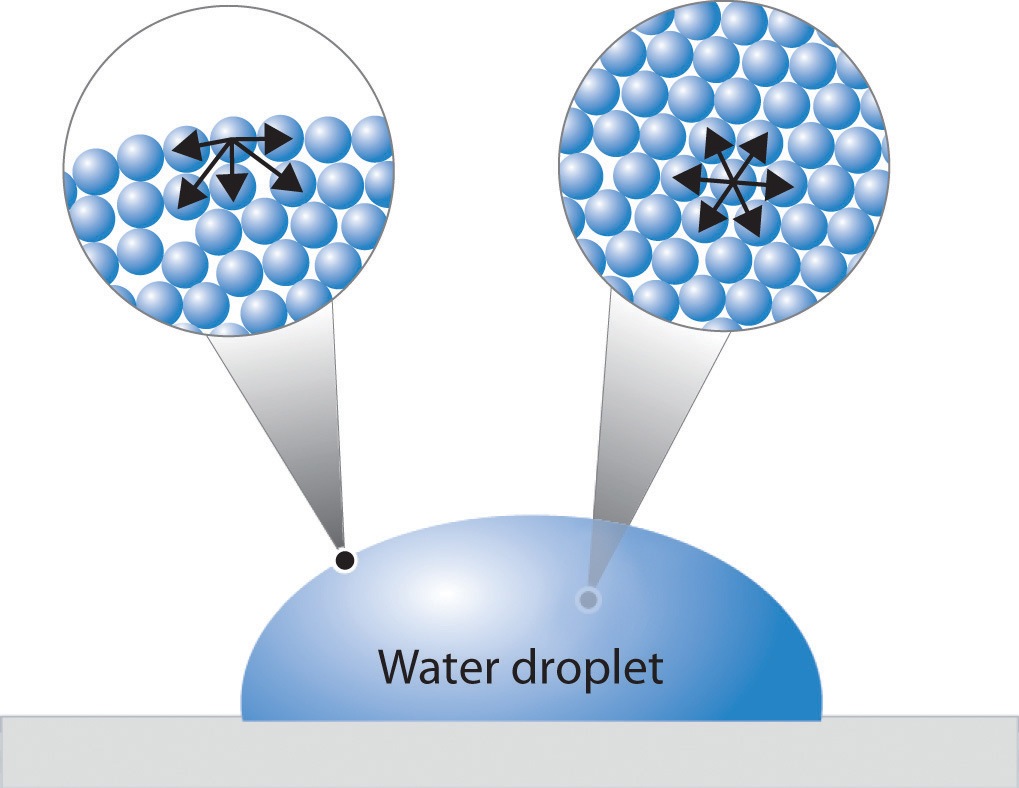
Surface Tension And Example . simply put, surface tension is the tendency of molecules of a liquid to be attracted. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The water molecules on the. The shape of the drops is caused by the surface tension of the water. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Surface tension results from a sharp change in the density between two adjoined phases or materials. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. examples of surface tension. the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
from chem.libretexts.org
The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. simply put, surface tension is the tendency of molecules of a liquid to be attracted. The shape of the drops is caused by the surface tension of the water. The water molecules on the. examples of surface tension. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension results from a sharp change in the density between two adjoined phases or materials.
Surface Tension Chemistry LibreTexts
Surface Tension And Example examples of surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The shape of the drops is caused by the surface tension of the water. When a water droplet forms, it tends to take on a spherical shape due to surface tension. simply put, surface tension is the tendency of molecules of a liquid to be attracted. The water molecules on the. the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Surface tension results from a sharp change in the density between two adjoined phases or materials. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. examples of surface tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Surface Tension And Example The water molecules on the. simply put, surface tension is the tendency of molecules of a liquid to be attracted. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. examples of surface tension. the surface tension is force per length and is measured by [n/m] and. Surface Tension And Example.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Surface Tension And Example the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The shape of the drops is caused by the surface tension of the water. Surface tension results from a sharp change in the density between two adjoined phases or materials. surface tension is the energy, or work, required to. Surface Tension And Example.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension And Example surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. Surface tension results from a sharp change in the density between two adjoined phases or materials. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. the surface tension. Surface Tension And Example.
From byjus.com
Explain the surface tension phenomenon with examples. Surface Tension And Example simply put, surface tension is the tendency of molecules of a liquid to be attracted. The shape of the drops is caused by the surface tension of the water. The water molecules on the. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When using a water dropper,. Surface Tension And Example.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Surface Tension And Example Surface tension results from a sharp change in the density between two adjoined phases or materials. The shape of the drops is caused by the surface tension of the water. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When using a water dropper, the water does not flow. Surface Tension And Example.
From www.slideserve.com
PPT Surface Tension PowerPoint Presentation, free download ID3106425 Surface Tension And Example surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. examples of surface tension. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. When a water droplet forms, it tends to take on a spherical shape due. Surface Tension And Example.
From www.youtube.com
Example Problem 1 Surface Tension YouTube Surface Tension And Example simply put, surface tension is the tendency of molecules of a liquid to be attracted. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The shape. Surface Tension And Example.
From depositphotos.com
Surface tension explanation vector illustration diagram Stock Vector Surface Tension And Example When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. simply put, surface tension is the tendency of molecules of a liquid to be attracted. The water molecules. Surface Tension And Example.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Surface Tension And Example simply put, surface tension is the tendency of molecules of a liquid to be attracted. The shape of the drops is caused by the surface tension of the water. Surface tension results from a sharp change in the density between two adjoined phases or materials. surface tension is the energy, or work, required to increase the surface area. Surface Tension And Example.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Surface Tension And Example When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. simply put, surface tension is the tendency of molecules of a liquid to be attracted. The only reason. Surface Tension And Example.
From thechemistrynotes.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences Surface Tension And Example Surface tension results from a sharp change in the density between two adjoined phases or materials. The shape of the drops is caused by the surface tension of the water. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. The water molecules on the. surface tension, property. Surface Tension And Example.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy Surface Tension And Example Surface tension results from a sharp change in the density between two adjoined phases or materials. When a water droplet forms, it tends to take on a spherical shape due to surface tension. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. examples of surface tension. When. Surface Tension And Example.
From en.wikipedia.org
FileSurface Tension Diagram.svg Wikipedia Surface Tension And Example The water molecules on the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The only reason the drop of water isn't completely spherical is that the force of gravity pulling. Surface Tension And Example.
From inspiritvr.com
Surface Tension Study Guide Inspirit Learning Inc Surface Tension And Example Surface tension results from a sharp change in the density between two adjoined phases or materials. examples of surface tension. simply put, surface tension is the tendency of molecules of a liquid to be attracted. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the. Surface Tension And Example.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Surface Tension And Example surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. simply put, surface tension is the tendency of molecules of a liquid to be attracted. The water molecules on the. surface tension is the energy, or work, required to increase the surface area of a liquid due to. Surface Tension And Example.
From www.slideserve.com
PPT Chapter 11 Liquids & Solids PowerPoint Presentation, free Surface Tension And Example The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension is force per length and is measured by [n/m] and is acting to stretch the. Surface Tension And Example.
From bulleintime.com
Surface Tension Definition And Example Surface Tension And Example surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The water molecules on the. simply put, surface tension is the tendency of molecules of a liquid to be attracted. When using a water dropper, the water does not flow in a continuous stream, but rather in a. Surface Tension And Example.
From www.researchgate.net
Surface tension and viscosity of various sample solutions Download Table Surface Tension And Example When a water droplet forms, it tends to take on a spherical shape due to surface tension. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. examples of surface tension. The shape of the drops is caused by the surface tension of the water. The water molecules. Surface Tension And Example.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Surface Tension And Example The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. The water molecules on the. Surface tension results from a sharp change in the density between two adjoined phases or materials. examples of surface tension. surface tension is the energy, or work, required to increase the surface area. Surface Tension And Example.
From byjus.com
Explain the surface tension phenomenon with examples. Surface Tension And Example When a water droplet forms, it tends to take on a spherical shape due to surface tension. examples of surface tension. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The shape of the drops is caused by the surface tension of the water. The water molecules on. Surface Tension And Example.
From www.thoughtco.com
Surface Tension Definition in Chemistry Surface Tension And Example Surface tension results from a sharp change in the density between two adjoined phases or materials. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The shape of the drops is caused by the surface tension of the water. The only reason the drop of water isn't completely. Surface Tension And Example.
From www.slideserve.com
PPT FLUID PROPERTIES Chapter 2 PowerPoint Presentation, free download Surface Tension And Example the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The water molecules on the. The shape of the drops is caused by the surface tension of the water. When using a water. Surface Tension And Example.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Surface Tension And Example simply put, surface tension is the tendency of molecules of a liquid to be attracted. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. examples of surface tension. The water molecules on the. When using a water dropper, the water does not flow in a continuous. Surface Tension And Example.
From www.youtube.com
Surface Tension, Viscosity and Capillary Action YouTube Surface Tension And Example examples of surface tension. the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension results from a sharp change in the density between two adjoined phases or materials. When using. Surface Tension And Example.
From byjus.com
What is Dimensional Formula of Surface Tension and its Derivation? Surface Tension And Example The shape of the drops is caused by the surface tension of the water. When a water droplet forms, it tends to take on a spherical shape due to surface tension. Surface tension results from a sharp change in the density between two adjoined phases or materials. When using a water dropper, the water does not flow in a continuous. Surface Tension And Example.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint Surface Tension And Example surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension results from a sharp change in the density between two adjoined phases or materials. examples of surface tension. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down. Surface Tension And Example.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Surface Tension And Example The water molecules on the. When a water droplet forms, it tends to take on a spherical shape due to surface tension. the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down. Surface Tension And Example.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Surface Tension And Example the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. simply put, surface tension is the tendency of molecules of a liquid to be attracted. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. The shape of the. Surface Tension And Example.
From www.biolinscientific.com
Surface tension of water Why is it so high? Surface Tension And Example When a water droplet forms, it tends to take on a spherical shape due to surface tension. The shape of the drops is caused by the surface tension of the water. The water molecules on the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. When using a. Surface Tension And Example.
From exolnazbp.blob.core.windows.net
Water Surface Tension With Temperature at Amber Holmes blog Surface Tension And Example examples of surface tension. The water molecules on the. The shape of the drops is caused by the surface tension of the water. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. When a water droplet forms, it tends to take on a spherical shape due to. Surface Tension And Example.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Surface Tension And Example The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. When using a water dropper, the water does not flow in a continuous stream, but rather in a series. Surface Tension And Example.
From www.adda247.com
Surface Tension Definition, Formula, Examples Surface Tension And Example surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. simply put, surface tension is the tendency of molecules of a liquid to be attracted. the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. The only reason the. Surface Tension And Example.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Surface Tension And Example the surface tension is force per length and is measured by [n/m] and is acting to stretch the surface. Surface tension results from a sharp change in the density between two adjoined phases or materials. When a water droplet forms, it tends to take on a spherical shape due to surface tension. The shape of the drops is caused. Surface Tension And Example.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Surface Tension And Example The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. Surface tension results from a sharp change in the density between two adjoined phases or materials. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. When a water. Surface Tension And Example.
From brainly.in
The dimensional formula for the surface tension? Brainly.in Surface Tension And Example surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. When a water droplet forms, it tends to take on a spherical shape due to surface tension. the surface. Surface Tension And Example.