What Is Gibbs Energy At Equilibrium . In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. Gibbs free energy 70 chemical potential is equal between different components in a system: In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. T is the temperature of the.
from www.slideserve.com
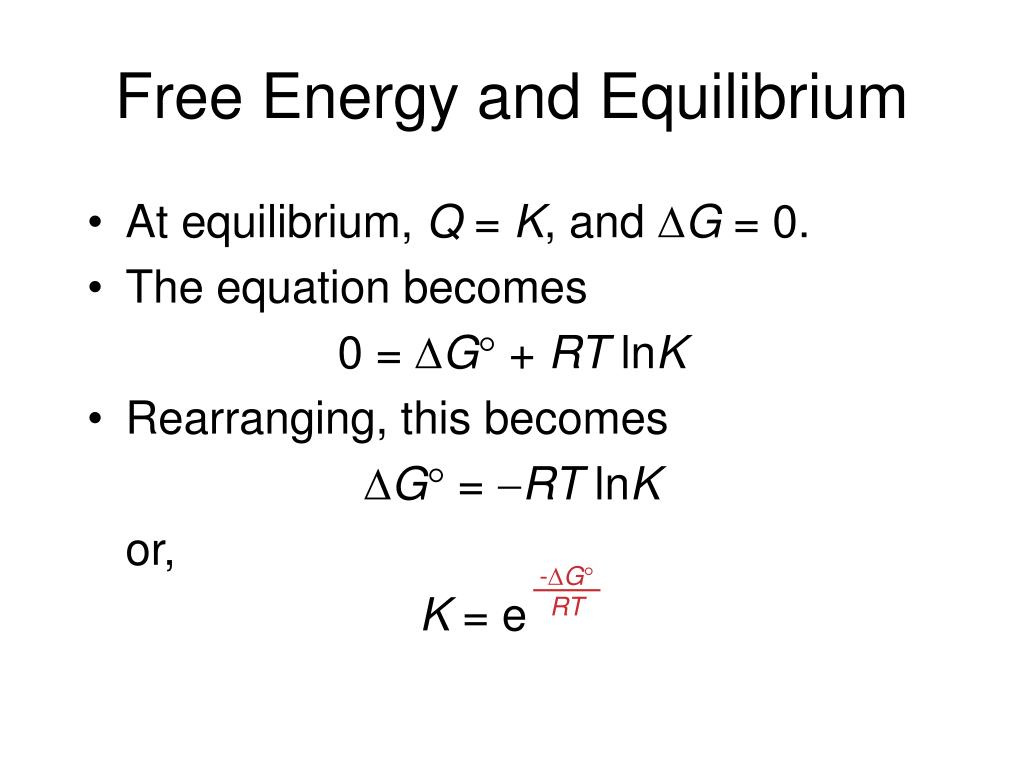
In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. T is the temperature of the. We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). Gibbs free energy 70 chemical potential is equal between different components in a system: In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q).
PPT Gibbs Free Energy PowerPoint Presentation, free download ID6582810
What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. T is the temperature of the. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. Gibbs free energy 70 chemical potential is equal between different components in a system:
From app.jove.com
Free Energy and Equilibrium Concept Cell Biology JoVe What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. T is the temperature of the. The change in free energy (δg) is the difference between the heat released during a process. What Is Gibbs Energy At Equilibrium.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID6125811 What Is Gibbs Energy At Equilibrium The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. We can predict whether a reaction will occur spontaneously by combining the. What Is Gibbs Energy At Equilibrium.
From www.numerade.com
SOLVED Using the same approach that we used in class to derive the What Is Gibbs Energy At Equilibrium We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). Gibbs free energy 70 chemical potential is equal between different components in a system: During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium. What Is Gibbs Energy At Equilibrium.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID6582810 What Is Gibbs Energy At Equilibrium We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. In this section, we explore the relationship between the free energy change. What Is Gibbs Energy At Equilibrium.
From www.youtube.com
Gibbs Energy and equilibrium constants YouTube What Is Gibbs Energy At Equilibrium T is the temperature of the. During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature. What Is Gibbs Energy At Equilibrium.
From www.researchgate.net
Equilibrium coexistence of ice and water. Illustration of potential What Is Gibbs Energy At Equilibrium Gibbs free energy 70 chemical potential is equal between different components in a system: T is the temperature of the. The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. During the late 1960's, researchers at nasa developed a general gibbs. What Is Gibbs Energy At Equilibrium.
From www.youtube.com
Gibbs Free Energy and Equilibrium YouTube What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. Gibbs free energy 70 chemical potential is equal between different components in. What Is Gibbs Energy At Equilibrium.
From mrschimomot.blogspot.com
Gibbs Free Energy Formula In Electrochemistry Mrschimomot What Is Gibbs Energy At Equilibrium We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. During the late 1960's, researchers at nasa developed a general gibbs minimization. What Is Gibbs Energy At Equilibrium.
From www.chemistrylearner.com
Gibbs Free Energy Definition, Equation, Unit, and Example What Is Gibbs Energy At Equilibrium Gibbs free energy 70 chemical potential is equal between different components in a system: During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free. What Is Gibbs Energy At Equilibrium.
From www.youtube.com
Gibbs Free Energy Entropy, Enthalpy & Equilibrium Constant K YouTube What Is Gibbs Energy At Equilibrium Gibbs free energy 70 chemical potential is equal between different components in a system: In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. T is the temperature of the. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q).. What Is Gibbs Energy At Equilibrium.
From www.savemyexams.com
Gibbs Free Energy & Equilibrium Constant (HL) HL IB Chemistry What Is Gibbs Energy At Equilibrium The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. Gibbs free energy 70 chemical potential is equal between different components in a system: We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of. What Is Gibbs Energy At Equilibrium.
From www.youtube.com
17.1 Equilibrium and Gibbs free energy (HL) YouTube What Is Gibbs Energy At Equilibrium T is the temperature of the. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Gibbs free energy 70 chemical potential is equal between different components in a system: We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system. What Is Gibbs Energy At Equilibrium.
From chem.libretexts.org
26.1 Equilibrium Results when Gibbs Energy is Minimized Chemistry What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). The change in free energy (δg) is the difference between the heat. What Is Gibbs Energy At Equilibrium.
From schoolbag.info
Figure 7.11. Gibbs Free Energy and Spontaneity A decrease in Gibbs free What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). The change in free energy (δg) is the difference between the heat released during a process and the heat released. What Is Gibbs Energy At Equilibrium.
From www.youtube.com
Electrochemistry 5 Nernst Equation Equilibrium Constant Gibb’s What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. T is the temperature of the. We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). The change in free energy (δg). What Is Gibbs Energy At Equilibrium.
From www.slideserve.com
PPT Gibbs Free Energy & Equilibrium Constants PowerPoint Presentation What Is Gibbs Energy At Equilibrium We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). Gibbs free energy 70 chemical potential is equal between different components in a system: The change in free energy (δg) is the difference between the heat released during a process and. What Is Gibbs Energy At Equilibrium.
From www.youtube.com
Gibbs Free Energy Equilibrium Constant, Enthalpy & Entropy Equations What Is Gibbs Energy At Equilibrium The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. T is the temperature of the. We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called. What Is Gibbs Energy At Equilibrium.
From www.slideserve.com
PPT Advanced Thermodynamics Note 12 ChemicalReaction Equilibria What Is Gibbs Energy At Equilibrium Gibbs free energy 70 chemical potential is equal between different components in a system: In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring. What Is Gibbs Energy At Equilibrium.
From www.masterorganicchemistry.com
Equilibrium and Energy Relationships Master Organic Chemistry What Is Gibbs Energy At Equilibrium Gibbs free energy 70 chemical potential is equal between different components in a system: During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. T is the temperature of the. In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. The change. What Is Gibbs Energy At Equilibrium.
From www.youtube.com
Chemical Equilibrium Using Gibbs Minimization Example YouTube What Is Gibbs Energy At Equilibrium The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. Gibbs free energy 70 chemical potential is equal between different components in a system: T is the temperature of the. In this section, we explore the relationship between the standard free. What Is Gibbs Energy At Equilibrium.
From questions-in.kunduz.com
GIBBS ENERGY CHANGE AND EQUILIBRIUM 97. Chemistry What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Gibbs free energy 70 chemical potential is equal between different components in a system: During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. In this section, we explore the. What Is Gibbs Energy At Equilibrium.
From www.youtube.com
Gibbs free energy and equilibrium YouTube What Is Gibbs Energy At Equilibrium Gibbs free energy 70 chemical potential is equal between different components in a system: We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the. What Is Gibbs Energy At Equilibrium.
From www.studypool.com
SOLUTION Gibbs Energy VS Equilibrium Studypool What Is Gibbs Energy At Equilibrium The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. Gibbs free energy 70 chemical potential is equal between different components in a system: In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the. What Is Gibbs Energy At Equilibrium.
From chem.libretexts.org
19.6 Gibbs Energy Change and Equilibrium Chemistry LibreTexts What Is Gibbs Energy At Equilibrium T is the temperature of the. The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. Gibbs free energy 70 chemical potential is equal between different components in a system: In this section, we explore the relationship between the standard free. What Is Gibbs Energy At Equilibrium.
From www.slideshare.net
IB Chemistry on Gibbs Free Energy, Equilibrium constant and Cell Pote… What Is Gibbs Energy At Equilibrium The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). During the. What Is Gibbs Energy At Equilibrium.
From www.slideserve.com
PPT 16 Chemical Equilibrium PowerPoint Presentation, free download What Is Gibbs Energy At Equilibrium We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. In this. What Is Gibbs Energy At Equilibrium.
From www.researchgate.net
1 Schematic plot of Gibbs free energy versus progress of reaction. The What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. T is the temperature of the. In this section, we explore the relationship between the standard free energy of reaction. What Is Gibbs Energy At Equilibrium.
From www.slideserve.com
PPT Gibbs Free Energy PowerPoint Presentation, free download ID6125811 What Is Gibbs Energy At Equilibrium During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. T is the temperature of the. We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). The change in free energy (δg). What Is Gibbs Energy At Equilibrium.
From www.slideserve.com
PPT Gibbs Free Energy & Equilibrium Constants PowerPoint Presentation What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). T is the temperature of the. The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. During the late 1960's,. What Is Gibbs Energy At Equilibrium.
From chem.libretexts.org
11.7 Gibbs Energy and Reaction Equilibrium Chemistry LibreTexts What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Gibbs free energy 70 chemical potential is equal between different components in a system: In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. The change in free energy (δg). What Is Gibbs Energy At Equilibrium.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system in a new state function called gibbs free energy (g). The change in free energy (δg) is the difference between. What Is Gibbs Energy At Equilibrium.
From www.youtube.com
GIBBS ENERGY CHANGE AND EQUILIBRIUM YouTube What Is Gibbs Energy At Equilibrium In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. Gibbs free energy 70 chemical potential is equal between different components in a system: The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a. What Is Gibbs Energy At Equilibrium.
From wisc.pb.unizin.org
Day 31 Le Châtelier’s Principle, Equilibrium and Gibbs Free Energy What Is Gibbs Energy At Equilibrium During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. In this section, we explore the relationship between the standard free energy of reaction (\(δg^o\)) and the equilibrium constant. T is the temperature of the. In this section, we explore the relationship between the free energy change of reaction (δg) and. What Is Gibbs Energy At Equilibrium.
From chem.libretexts.org
19.6 Gibbs Energy Change and Equilibrium Chemistry LibreTexts What Is Gibbs Energy At Equilibrium T is the temperature of the. In this section, we explore the relationship between the free energy change of reaction (δg) and the instantaneous reaction quotient (q). Gibbs free energy 70 chemical potential is equal between different components in a system: We can predict whether a reaction will occur spontaneously by combining the entropy, enthalpy, and temperature of a system. What Is Gibbs Energy At Equilibrium.
From chem.libretexts.org
11.7 Gibbs Energy and Reaction Equilibrium Chemistry LibreTexts What Is Gibbs Energy At Equilibrium The change in free energy (δg) is the difference between the heat released during a process and the heat released for the same process occurring in a reversible manner. T is the temperature of the. During the late 1960's, researchers at nasa developed a general gibbs minimization approach for finding the equilibrium composition of. In this section, we explore the. What Is Gibbs Energy At Equilibrium.