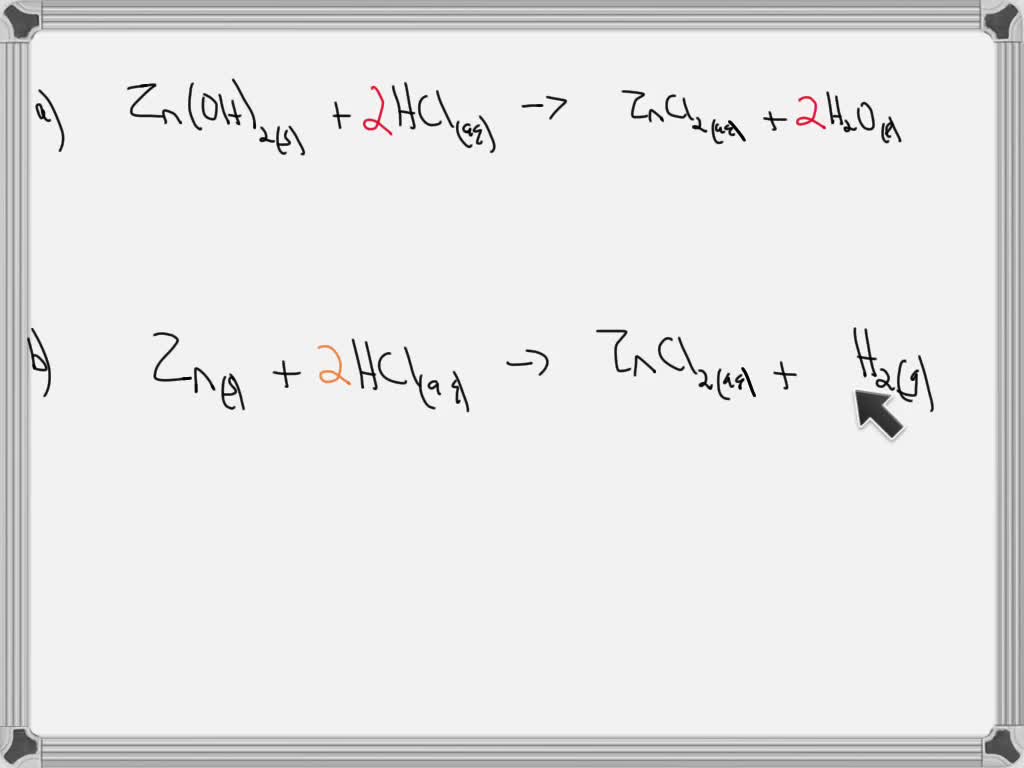Zinc Chloride Decomposition . The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Decomposition reactions involve a single compound breaking down into two or more products. This compound reacts with ammonia to form complexes. Erg 2024, guide 154 (zinc chloride, solution; Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and. Examples include zn(nh 3) 4 cl 2. The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1.
from www.numerade.com
Examples include zn(nh 3) 4 cl 2. Decomposition reactions involve a single compound breaking down into two or more products. The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and. This compound reacts with ammonia to form complexes. Erg 2024, guide 154 (zinc chloride, solution;
SOLVED Describe how to prepare zinc chloride by (a) an acid base
Zinc Chloride Decomposition Examples include zn(nh 3) 4 cl 2. Decomposition reactions involve a single compound breaking down into two or more products. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. This compound reacts with ammonia to form complexes. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Examples include zn(nh 3) 4 cl 2. Erg 2024, guide 154 (zinc chloride, solution;
From www.numerade.com
SOLVED The of zinc carbonate, \mathrm{ZnCO}_{3}(\mathrm Zinc Chloride Decomposition Erg 2024, guide 154 (zinc chloride, solution; Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Examples include zn(nh 3) 4 cl 2. Decomposition reactions involve a single compound breaking down into two or more products. This compound reacts with ammonia to form complexes. Zncl2 = zn + cl2 is a decomposition. Zinc Chloride Decomposition.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Chloride Decomposition The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). This compound reacts with ammonia to form complexes. Erg 2024, guide 154 (zinc chloride, solution; Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride. Zinc Chloride Decomposition.
From www.numerade.com
SOLVED A displacement reaction takes place when calcium is placed in a Zinc Chloride Decomposition This compound reacts with ammonia to form complexes. Examples include zn(nh 3) 4 cl 2. Decomposition reactions involve a single compound breaking down into two or more products. Erg 2024, guide 154 (zinc chloride, solution; The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Zncl2 = zn + cl2 is a decomposition reaction. Zinc Chloride Decomposition.
From www.numerade.com
SOLVED Describe how to prepare zinc chloride by (a) an acid base Zinc Chloride Decomposition Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Erg 2024, guide 154 (zinc chloride, solution; Decomposition reactions involve a single compound breaking down into. Zinc Chloride Decomposition.
From www.researchgate.net
Reaction scheme showing the thermal of zinc (II Zinc Chloride Decomposition Erg 2024, guide 154 (zinc chloride, solution; The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Decomposition reactions involve a single compound breaking down into two or more products. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Zncl2 = zn +. Zinc Chloride Decomposition.
From www.pw.live
Zinc Chloride Formula Zinc Chloride Decomposition The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. This compound reacts with ammonia to form complexes. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Decomposition reactions involve a single compound breaking down into two or more products. Examples include zn(nh 3) 4 cl. Zinc Chloride Decomposition.
From www.911metallurgist.com
How to Recover Zinc from Zinc Chloride by Electrolysis Zinc Chloride Decomposition Erg 2024, guide 154 (zinc chloride, solution; The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Decomposition reactions involve a single compound breaking down into two or more products. Examples include zn(nh 3) 4 cl 2.. Zinc Chloride Decomposition.
From www.youtube.com
How to Balance Zn(OH)2 = ZnO + H2O (Zinc hydroxide YouTube Zinc Chloride Decomposition This compound reacts with ammonia to form complexes. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Examples include zn(nh 3) 4 cl 2. The ph of an aqueous solution of zinc. Zinc Chloride Decomposition.
From achs-prod.acs.org
Mechanism of Zinc Ammine Borohydride A FirstPrinciples Zinc Chloride Decomposition Examples include zn(nh 3) 4 cl 2. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). This compound reacts with ammonia to form complexes. Erg 2024, guide 154 (zinc chloride, solution; Decomposition reactions involve a single compound breaking down into two or more products. The ph of an aqueous. Zinc Chloride Decomposition.
From www.x-mol.com
Effects of zinc chloridesilicone oil treatment on wood dimensional Zinc Chloride Decomposition The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Examples include zn(nh 3) 4 cl 2. This compound reacts with ammonia to form complexes. Decomposition reactions involve a single compound breaking down into two or more products. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride. Zinc Chloride Decomposition.
From www.nagwa.com
Question Video Identifying the Correct Chemical Equation for the Zinc Chloride Decomposition The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Examples include zn(nh 3) 4 cl 2. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). This compound reacts with ammonia to form complexes. Erg 2024, guide 154 (zinc chloride, solution; Zncl2 =. Zinc Chloride Decomposition.
From www.bossgoo.com
Physical Properties Of Zinc Chloride Structure, High Zinc Chloride Decomposition Erg 2024, guide 154 (zinc chloride, solution; This compound reacts with ammonia to form complexes. The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Examples include zn(nh 3) 4 cl 2. Decomposition reactions involve a single compound breaking down into two or more products. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride. Zinc Chloride Decomposition.
From dokumen.tips
(PDF) Fused zinc chloride Part IV Reactions of some transition metal Zinc Chloride Decomposition Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and. Erg 2024, guide 154 (zinc chloride, solution; Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Examples include zn(nh 3) 4 cl 2. The ph. Zinc Chloride Decomposition.
From www.chegg.com
Solved REPORT SHEET Chemical Formulas A, Zinc Chloride 1. Zinc Chloride Decomposition Erg 2024, guide 154 (zinc chloride, solution; Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. This compound reacts with ammonia to form complexes. Examples include zn(nh 3) 4 cl 2. Decomposition reactions involve a single compound breaking down into two or more products. The dsc thermogram of zinc chloride showed a. Zinc Chloride Decomposition.
From www.researchgate.net
(PDF) Catalytic of cumene hydroperoxide into phenol and Zinc Chloride Decomposition Examples include zn(nh 3) 4 cl 2. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. This compound reacts with ammonia to form complexes. Decomposition reactions involve a single compound breaking down. Zinc Chloride Decomposition.
From studylib.net
File Zinc Chloride Decomposition The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and.. Zinc Chloride Decomposition.
From pubs.rsc.org
163. The influence of psubstituents on the of zinc Zinc Chloride Decomposition Erg 2024, guide 154 (zinc chloride, solution; Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Decomposition reactions involve a single compound breaking down into two or more products. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Zncl2 = zn. Zinc Chloride Decomposition.
From www.bossgoo.com
Zinc Chloride Distributor, Zinc Chloride Zinc Chloride Zinc Chloride Decomposition The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. This compound reacts with ammonia to form complexes. Erg 2024, guide 154 (zinc chloride, solution; Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Zncl2 = zn + cl2 is a decomposition reaction where one mole. Zinc Chloride Decomposition.
From www.youtube.com
Double displacement of ZnSO4 + BaCl2 Zinc sulphate + Barium chloride Zinc Chloride Decomposition Decomposition reactions involve a single compound breaking down into two or more products. This compound reacts with ammonia to form complexes. Examples include zn(nh 3) 4 cl 2. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a. Zinc Chloride Decomposition.
From pubs.rsc.org
118. The of zinc chloride double salts of diazonium Zinc Chloride Decomposition Examples include zn(nh 3) 4 cl 2. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and. Decomposition reactions involve a single compound. Zinc Chloride Decomposition.
From www.youtube.com
Chemistry Experiment How To Make Zinc Acetate Full HD video YouTube Zinc Chloride Decomposition The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Examples include zn(nh 3) 4 cl 2. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with.. Zinc Chloride Decomposition.
From www.researchgate.net
(PDF) Effects of zinc chloridesilicone oil treatment on wood Zinc Chloride Decomposition The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Erg 2024, guide 154 (zinc chloride, solution; The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with.. Zinc Chloride Decomposition.
From www.researchgate.net
What is the proper way to prepare a zinc chloride containing buffer for Zinc Chloride Decomposition Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1.. Zinc Chloride Decomposition.
From www.academia.edu
(PDF) The products of the zinc chloridepromoted of Zinc Chloride Decomposition The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. This compound reacts with ammonia to form complexes. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride. Zinc Chloride Decomposition.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Chloride Decomposition Examples include zn(nh 3) 4 cl 2. Erg 2024, guide 154 (zinc chloride, solution; Decomposition reactions involve a single compound breaking down into two or more products. This compound reacts with ammonia to form complexes. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc. Zinc Chloride Decomposition.
From www.researchgate.net
Cytotoxicity of zinc chloride and a zincerythritol mixture. The Zinc Chloride Decomposition Decomposition reactions involve a single compound breaking down into two or more products. Erg 2024, guide 154 (zinc chloride, solution; Examples include zn(nh 3) 4 cl 2. The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with.. Zinc Chloride Decomposition.
From www.chegg.com
Solved A. Zinc Chloride 1. Mass of evaporating dish and zinc Zinc Chloride Decomposition The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. This compound reacts with ammonia to form complexes. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride. Zinc Chloride Decomposition.
From avopix.com
Reaction Infographic Diagram with Royalty Free Stock Zinc Chloride Decomposition This compound reacts with ammonia to form complexes. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and. Examples include zn(nh 3) 4 cl 2. The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Erg 2024,. Zinc Chloride Decomposition.
From klabxtjsm.blob.core.windows.net
Potassium Hydroxide And Zinc Equation at James Roden blog Zinc Chloride Decomposition The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Decomposition reactions involve a single compound breaking down into two or more products. Examples include zn(nh 3) 4 cl 2. Zncl2 = zn +. Zinc Chloride Decomposition.
From www.toppr.com
Identify the type of reactions taking place in each of the following Zinc Chloride Decomposition The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Examples include zn(nh 3) 4 cl 2. Erg 2024, guide 154 (zinc chloride, solution; Decomposition reactions involve a single compound breaking down into two or more products. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a. Zinc Chloride Decomposition.
From www.numerade.com
SOLVED Question 14 (5 points) Calculate how many moles of zinc Zinc Chloride Decomposition This compound reacts with ammonia to form complexes. The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Decomposition reactions involve a single compound breaking down into two or more products. The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Zncl2 = zn. Zinc Chloride Decomposition.
From www.shutterstock.com
122 Zinc Chloride Images, Stock Photos & Vectors Shutterstock Zinc Chloride Decomposition The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Decomposition reactions involve a single compound breaking down into two or more products. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl 2] decomposes into one mole of solid zinc [zn] and.. Zinc Chloride Decomposition.
From www.numerade.com
SOLVEDThe reaction of a drycell battery may be represented as follows Zinc Chloride Decomposition This compound reacts with ammonia to form complexes. Decomposition reactions involve a single compound breaking down into two or more products. Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Erg 2024, guide 154 (zinc chloride, solution; Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc. Zinc Chloride Decomposition.
From www.researchgate.net
(PDF) Surface Morphology and Microstructure of Zinc Deposit From Zinc Chloride Decomposition Erg 2024, guide 154 (zinc chloride, solution; Decomposition reactions involve a single compound breaking down into two or more products. Examples include zn(nh 3) 4 cl 2. The ph of an aqueous solution of zinc chloride with a concentration of 6m is 1. Zncl2 = zn + cl2 is a decomposition reaction where one mole of aqueous zinc chloride [zncl. Zinc Chloride Decomposition.
From www.researchgate.net
Effect of zinc chloride concentration on cellulose dissolution Zinc Chloride Decomposition The dsc thermogram of zinc chloride showed a small endothermic inflation (δh fusion) and a larger exothermic peak (δh decomposition). Zinc chloride hydroxide monohydrate or more accurately pentazinc dichloride octahydroxide monohydrate is a zinc hydroxy compound with. Erg 2024, guide 154 (zinc chloride, solution; Decomposition reactions involve a single compound breaking down into two or more products. The ph of. Zinc Chloride Decomposition.