Usp Assay Specifications . This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. What you need to know and how to find proven services. Test a and test b (added) assay. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. Potential new chapter control of organic impurities in drug substances. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Chloride (deleted) and organic impurities (added) specific tests. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy.
from www.waters.com
This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. What you need to know and how to find proven services. Potential new chapter control of organic impurities in drug substances. Test a and test b (added) assay. Chloride (deleted) and organic impurities (added) specific tests.
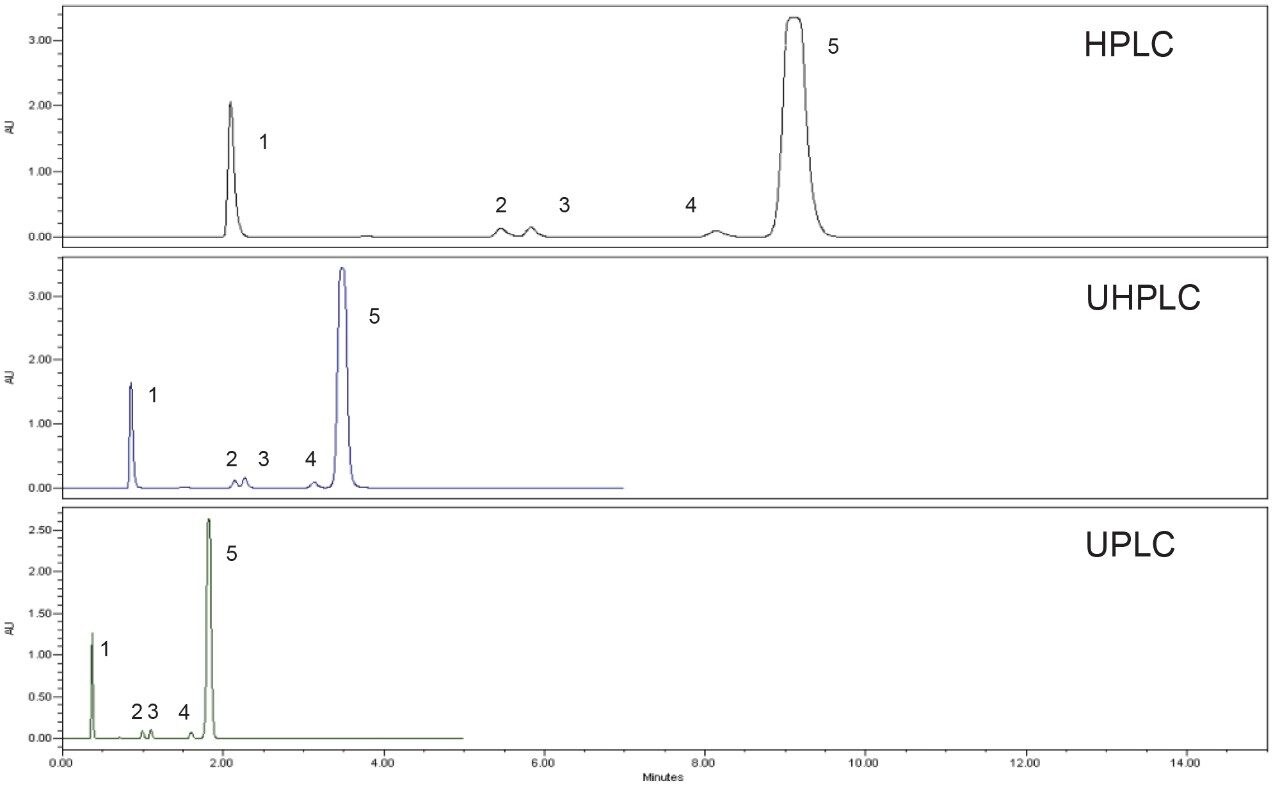
Scaling of a USP Assay for Quetiapine Fumarate Across Different Liquid
Usp Assay Specifications Test a and test b (added) assay. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Chloride (deleted) and organic impurities (added) specific tests. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Potential new chapter control of organic impurities in drug substances. What you need to know and how to find proven services. Test a and test b (added) assay.
From www.waters.com
Transfer of an Isocratic USP Assay from an Agilent 1100 Series LC Usp Assay Specifications Chloride (deleted) and organic impurities (added) specific tests. Potential new chapter control of organic impurities in drug substances. Test a and test b (added) assay. What you need to know and how to find proven services. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Usp currently offers more than. Usp Assay Specifications.
From www.mac-mod.com
Amoxicillin USP Assay MACMOD Analytical Usp Assay Specifications Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. What you need to know and how to find proven services. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances,. Usp Assay Specifications.
From www.avancebio.com
USP Assays Avance Biosciences Usp Assay Specifications Chloride (deleted) and organic impurities (added) specific tests. Test a and test b (added) assay. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. What you need to know and how to find. Usp Assay Specifications.
From dokumen.tips
(PDF) Method Validation Based on ICH Guidelines of a USP Assay Usp Assay Specifications What you need to know and how to find proven services. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Chloride (deleted) and organic impurities (added) specific tests. Potential new chapter control of. Usp Assay Specifications.
From dokumen.tips
(PDF) S1529, Sodium Chloride, USP, EP Spectrum Chemical · synonyms Usp Assay Specifications What you need to know and how to find proven services. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Test a and test b (added) assay. Chloride (deleted) and organic impurities (added) specific tests. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,.. Usp Assay Specifications.
From www.slideserve.com
PPT USP Reference Standards for Biologics PowerPoint Presentation Usp Assay Specifications What you need to know and how to find proven services. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Chloride (deleted) and organic impurities (added) specific tests. Potential new chapter control of organic impurities in drug substances. Test a and test b (added) assay. This advanced guide will help. Usp Assay Specifications.
From www.waters.com
Transferring the USP Assay of Zidovudine Using CORTECS 2.7 μm Columns Usp Assay Specifications This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. Assurance of the quality of pharmaceuticals is accomplished by. Usp Assay Specifications.
From www.waters.com
Transfer of an Isocratic USP Assay from an Agilent 1100 Series LC Usp Assay Specifications What you need to know and how to find proven services. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Test a and test b (added) assay. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. Chloride (deleted) and organic impurities (added) specific. Usp Assay Specifications.
From www.researchgate.net
USP11 stabilized TRAF3 by deubiquitination. a, b) Western blot assay Usp Assay Specifications This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Potential new chapter control of organic impurities in drug substances. Chloride (deleted) and organic impurities (added) specific tests. What you need to know and how to find proven services. Test a and test b (added) assay. Assurance of the quality of pharmaceuticals is. Usp Assay Specifications.
From www.mac-mod.com
USP Assay Hydrocortisone Cream Usp Assay Specifications Chloride (deleted) and organic impurities (added) specific tests. What you need to know and how to find proven services. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Potential new chapter control of. Usp Assay Specifications.
From www.mac-mod.com
Paclitaxel Assay (USP Monograph Method) Usp Assay Specifications Potential new chapter control of organic impurities in drug substances. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Test a and test b (added) assay. Chloride (deleted) and organic impurities (added) specific tests. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation. Usp Assay Specifications.
From www.usp.org
USP Monograph and Reference Standards Development Step by Step USP Usp Assay Specifications Test a and test b (added) assay. What you need to know and how to find proven services. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Chloride (deleted) and organic impurities (added) specific tests.. Usp Assay Specifications.
From www.scribd.com
General Chapters 11 Usp Reference Standards PDF Chemical Usp Assay Specifications Test a and test b (added) assay. Potential new chapter control of organic impurities in drug substances. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. What you need to know and how to find proven services. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust. Usp Assay Specifications.
From www.waters.com
Modernizing USP Melatonin Monograph Assay and Impurities Methods for Usp Assay Specifications Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. What you need to know and how to find proven services. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances,. Usp Assay Specifications.
From microbe-investigations.com
USP 62 Microbiological Testing of NonSterile Products Usp Assay Specifications Test a and test b (added) assay. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. Chloride (deleted) and organic impurities (added) specific tests. Potential new chapter control of organic impurities in drug substances. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Assurance of. Usp Assay Specifications.
From studylib.net
Full USP Specification for Liquid Chromatography Columns Usp Assay Specifications Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. Test a and test b (added) assay. What you need to know and how to find proven services. This advanced guide will help you properly. Usp Assay Specifications.
From www.waters.com
Transfer of an Isocratic USP Assay from an Agilent 1100 Series LC Usp Assay Specifications Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Potential new chapter control of organic impurities in drug. Usp Assay Specifications.
From www.slideserve.com
PPT Biological Assays PowerPoint Presentation, free download ID2946199 Usp Assay Specifications This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Potential new chapter control of organic impurities in drug substances. Test a and test b (added) assay. Chloride (deleted) and organic impurities (added) specific. Usp Assay Specifications.
From www.yumpu.com
COLUMN SELECTION BY USP SPECIFICATIONS Usp Assay Specifications This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. What you need to know and how to find proven services. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Test a and test b (added) assay. Potential new chapter control of organic impurities. Usp Assay Specifications.
From www.scribd.com
USP 36 Assay Accuracy And Precision Usp Assay Specifications Potential new chapter control of organic impurities in drug substances. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Test a and test b (added) assay. What you need to know and how to find proven services. Chloride (deleted) and organic impurities (added) specific tests. This chapter summarizes these procedures. Usp Assay Specifications.
From www.waters.com
Modernizing USP Melatonin Monograph Assay and Impurities Methods for Usp Assay Specifications This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Test a and test b (added) assay. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Potential new. Usp Assay Specifications.
From www.youtube.com
Main Types of USP Testing Specifications Explained YouTube Usp Assay Specifications Potential new chapter control of organic impurities in drug substances. Test a and test b (added) assay. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. What you need to know and how to find proven services. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients,. Usp Assay Specifications.
From www.researchgate.net
Comparison of developed method and USP method based on assay Usp Assay Specifications This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Chloride (deleted) and organic impurities (added) specific tests. Test a and test b (added) assay. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. What you need to know and how to. Usp Assay Specifications.
From www.waters.com
USP Analysis of Glimepiride on an Alliance HPLC System Modernization Usp Assay Specifications Potential new chapter control of organic impurities in drug substances. Test a and test b (added) assay. What you need to know and how to find proven services. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. This advanced guide will help you properly assess usp testing specifications then best plan your testing. Usp Assay Specifications.
From www.scribd.com
Rivaroxaban USP Method Evaluation Report PDF Chromatography Usp Assay Specifications Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. What you need to know and how to find proven services. Chloride (deleted) and organic impurities (added) specific tests. Potential new chapter control of organic impurities in drug substances. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including. Usp Assay Specifications.
From www.waters.com
Scaling of a USP Assay for Quetiapine Fumarate Across Different Liquid Usp Assay Specifications This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Potential new chapter control of organic impurities in drug substances. Test a and test b (added) assay. This advanced guide will help you. Usp Assay Specifications.
From dokumen.tips
(PDF) Optimization of the USP assay for hyaluronidase DOKUMEN.TIPS Usp Assay Specifications Chloride (deleted) and organic impurities (added) specific tests. Test a and test b (added) assay. Potential new chapter control of organic impurities in drug substances. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. Assurance of. Usp Assay Specifications.
From www.reactionbiology.com
USP20 Deubiquitination Assay Service Reaction Biology Usp Assay Specifications This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Chloride (deleted) and organic impurities (added) specific tests. What you need to know and how to find proven services. Test a and test b (added) assay. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,.. Usp Assay Specifications.
From www.waters.com
The USP Method for Topiramate Assay using UPLC and Refractive Index Usp Assay Specifications Chloride (deleted) and organic impurities (added) specific tests. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Test a and test b (added) assay. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia. Usp Assay Specifications.
From www.honeymanlaboratories.com
Water for Injection (WFI) Analysis Usp Assay Specifications Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Test a and test b (added) assay. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. What you. Usp Assay Specifications.
From www.yumpu.com
Validation of Changes to the USP Assay Method for Ibuprofen Tablets Usp Assay Specifications This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. Potential new chapter control of organic impurities in drug substances. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. What you need to know and how to find proven services. Usp currently offers more. Usp Assay Specifications.
From dailymed.nlm.nih.gov
Oxygen USP Bulk Liquid Usp Assay Specifications Potential new chapter control of organic impurities in drug substances. Chloride (deleted) and organic impurities (added) specific tests. Test a and test b (added) assay. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. This. Usp Assay Specifications.
From www.slideserve.com
PPT Biological Assays PowerPoint Presentation, free download ID2946199 Usp Assay Specifications Potential new chapter control of organic impurities in drug substances. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Usp currently offers more than 3,500 reference standards—highly characterized specimens of drug substances, excipients, food ingredients,. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological. Usp Assay Specifications.
From www.scribd.com
Water Usp PDF Purified Water Water Purification Usp Assay Specifications Chloride (deleted) and organic impurities (added) specific tests. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. Test a and test b (added) assay. This advanced guide will help you properly assess usp testing specifications then best plan your testing strategy. This chapter summarizes these procedures for the antibiotics recognized. Usp Assay Specifications.
From www.scribd.com
Comparison Bet. US FDA, USP & ICH Guidelines Verification And Usp Assay Specifications Chloride (deleted) and organic impurities (added) specific tests. Test a and test b (added) assay. This chapter summarizes these procedures for the antibiotics recognized in this pharmacopeia for which microbiological assay remains the. Assurance of the quality of pharmaceuticals is accomplished by combining a number of practices, including robust formulation design, validation,. This advanced guide will help you properly assess. Usp Assay Specifications.