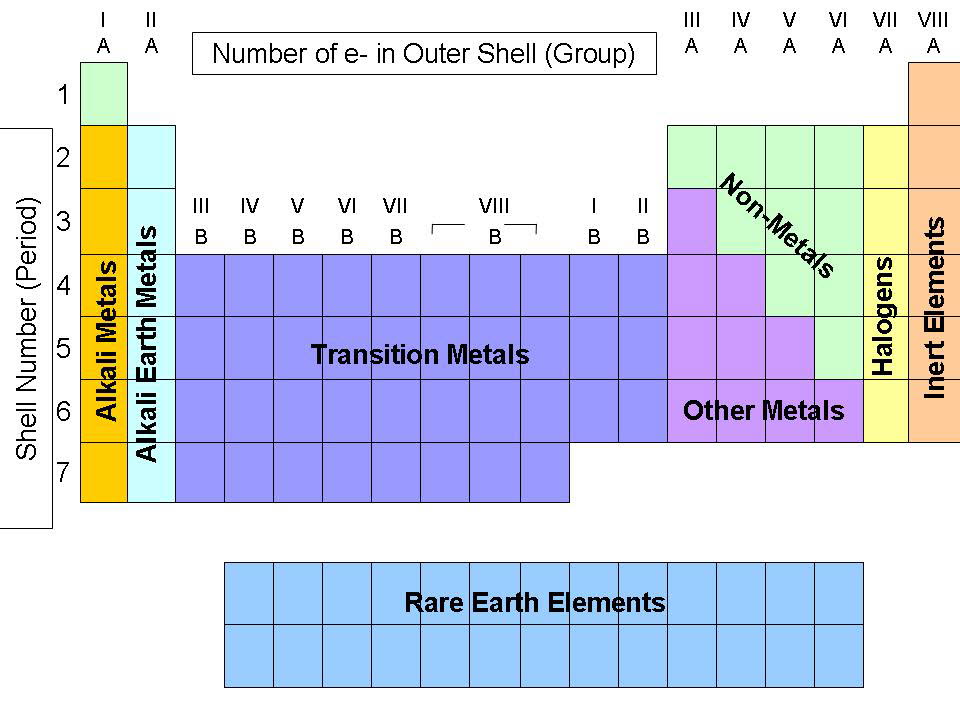
Halogens And Alkali Metals . losing their outer electron will mean they form ions with a +1 charge e.g. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. Li +, na +, k +, rb +, cs +. The elements in group 7 are known as halogens. The heavier chalcogens (group 16) to produce metal chalcogenides; when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. this section describes the chemistry of halogens with the main group elements such as the alkali metals,. Fluorine and chlorine are gases. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. Bromine is one of only two liquid. the alkali metals react with halogens (group 17) to form ionic halides; The properties of alkali metals. alkali metals readily react with halogens and, in fact, they react violently under certain conditions.
from new-periodic11.blogspot.com
alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. The elements in group 7 are known as halogens. The properties of alkali metals. Li +, na +, k +, rb +, cs +. Bromine is one of only two liquid. The heavier chalcogens (group 16) to produce metal chalcogenides; alkali metals readily react with halogens and, in fact, they react violently under certain conditions. Fluorine and chlorine are gases. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. this section describes the chemistry of halogens with the main group elements such as the alkali metals,.
NEW PERIODIC TABLE ALKALI METALS AND HALOGENS Periodic
Halogens And Alkali Metals Bromine is one of only two liquid. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. Li +, na +, k +, rb +, cs +. the alkali metals react with halogens (group 17) to form ionic halides; The properties of alkali metals. The elements in group 7 are known as halogens. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. The heavier chalcogens (group 16) to produce metal chalcogenides; this section describes the chemistry of halogens with the main group elements such as the alkali metals,. losing their outer electron will mean they form ions with a +1 charge e.g. Bromine is one of only two liquid. Fluorine and chlorine are gases. alkali metals readily react with halogens and, in fact, they react violently under certain conditions.
From www.slideshare.net
Chemistry of alkali and alkaline earth metals and halogen compounds Halogens And Alkali Metals losing their outer electron will mean they form ions with a +1 charge e.g. The elements in group 7 are known as halogens. Fluorine and chlorine are gases. The properties of alkali metals. when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. The heavier chalcogens (group. Halogens And Alkali Metals.
From elchoroukhost.net
Alkali Metals Halogens Periodic Table Elcho Table Halogens And Alkali Metals Fluorine and chlorine are gases. The elements in group 7 are known as halogens. this section describes the chemistry of halogens with the main group elements such as the alkali metals,. when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. The heavier chalcogens (group 16) to. Halogens And Alkali Metals.
From elchoroukhost.net
Alkali Metals Halogens Periodic Table Elcho Table Halogens And Alkali Metals Bromine is one of only two liquid. The properties of alkali metals. the alkali metals react with halogens (group 17) to form ionic halides; And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. The heavier chalcogens (group 16) to produce metal chalcogenides; Fluorine and chlorine are gases. this section describes the chemistry. Halogens And Alkali Metals.
From periodictableguide.com
How are the Elements Arranged in the Modern Periodic Table? Halogens And Alkali Metals The properties of alkali metals. Li +, na +, k +, rb +, cs +. alkali metals readily react with halogens and, in fact, they react violently under certain conditions. Fluorine and chlorine are gases. the alkali metals react with halogens (group 17) to form ionic halides; And oxygen to form compounds, whose stoichiometry depends on the size. Halogens And Alkali Metals.
From elchoroukhost.net
Families On The Periodic Table Of Elements Elcho Table Halogens And Alkali Metals this section describes the chemistry of halogens with the main group elements such as the alkali metals,. The elements in group 7 are known as halogens. losing their outer electron will mean they form ions with a +1 charge e.g. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. The heavier chalcogens. Halogens And Alkali Metals.
From assign.unaux.com
Periodic properties of alkali metals and halogens. assign Halogens And Alkali Metals Bromine is one of only two liquid. losing their outer electron will mean they form ions with a +1 charge e.g. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. alkali metals readily react with halogens and, in fact, they react violently under certain conditions. Li +, na +, k +, rb. Halogens And Alkali Metals.
From sciencenotes.org
Halogen Elements List and Facts Halogens And Alkali Metals Li +, na +, k +, rb +, cs +. when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. The heavier chalcogens (group 16) to. Halogens And Alkali Metals.
From utedzz.blogspot.com
Periodic Table With Halogens Alkali Metals Periodic Table Timeline Halogens And Alkali Metals losing their outer electron will mean they form ions with a +1 charge e.g. Li +, na +, k +, rb +, cs +. The heavier chalcogens (group 16) to produce metal chalcogenides; alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. this section describes. Halogens And Alkali Metals.
From www.pinterest.com
Comparing halogen reactivity trends with those of the alkali metals Halogens And Alkali Metals the alkali metals react with halogens (group 17) to form ionic halides; alkali metals readily react with halogens and, in fact, they react violently under certain conditions. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. The elements in group 7 are known as halogens. Li +, na +, k +, rb. Halogens And Alkali Metals.
From www.slideserve.com
PPT The Periodic Table! PowerPoint Presentation, free download ID Halogens And Alkali Metals losing their outer electron will mean they form ions with a +1 charge e.g. Li +, na +, k +, rb +, cs +. when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. And oxygen to form compounds, whose stoichiometry depends on the size of the. Halogens And Alkali Metals.
From www.youtube.com
The Alkali Metals and the Halogens YouTube Halogens And Alkali Metals losing their outer electron will mean they form ions with a +1 charge e.g. The elements in group 7 are known as halogens. The heavier chalcogens (group 16) to produce metal chalcogenides; The properties of alkali metals. when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are.. Halogens And Alkali Metals.
From www.youtube.com
8.9 Some Examples of Periodic Chemical Behavior The Alkali Metals, the Halogens And Alkali Metals And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. Li +, na +, k +, rb +, cs +. this section describes the chemistry of halogens with the main group elements such as the alkali metals,. Bromine is one of only two liquid. alkali metals readily react with halogens and, in fact,. Halogens And Alkali Metals.
From new-periodic11.blogspot.com
NEW PERIODIC TABLE ALKALI METALS AND HALOGENS Periodic Halogens And Alkali Metals Bromine is one of only two liquid. Li +, na +, k +, rb +, cs +. The heavier chalcogens (group 16) to produce metal chalcogenides; alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. The properties of alkali metals. alkali metals readily react with halogens. Halogens And Alkali Metals.
From www.sliderbase.com
The groups, and electron dot diagrams Presentation Chemistry Halogens And Alkali Metals alkali metals readily react with halogens and, in fact, they react violently under certain conditions. Bromine is one of only two liquid. the alkali metals react with halogens (group 17) to form ionic halides; losing their outer electron will mean they form ions with a +1 charge e.g. Li +, na +, k +, rb +, cs. Halogens And Alkali Metals.
From ar.inspiredpencil.com
Periodic Table Of Elements With Alkali Metals Halogens And Alkali Metals losing their outer electron will mean they form ions with a +1 charge e.g. this section describes the chemistry of halogens with the main group elements such as the alkali metals,. Li +, na +, k +, rb +, cs +. The properties of alkali metals. The elements in group 7 are known as halogens. the alkali. Halogens And Alkali Metals.
From elchoroukhost.net
Alkali Metals Halogens Periodic Table Elcho Table Halogens And Alkali Metals The heavier chalcogens (group 16) to produce metal chalcogenides; And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. the alkali metals react with halogens (group 17) to form ionic halides; alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. The. Halogens And Alkali Metals.
From utedzz.blogspot.com
Periodic Table With Halogens Alkali Metals Periodic Table Timeline Halogens And Alkali Metals The heavier chalcogens (group 16) to produce metal chalcogenides; alkali metals readily react with halogens and, in fact, they react violently under certain conditions. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. Bromine is one of only two liquid. The elements in group 7 are. Halogens And Alkali Metals.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID6080292 Halogens And Alkali Metals when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. The heavier chalcogens (group 16) to produce metal chalcogenides; the alkali metals react with halogens (group 17) to form ionic halides; Li +, na +, k +, rb +, cs +. The elements in group 7 are. Halogens And Alkali Metals.
From www.scribd.com
Comparison of Properties of Alkali Metals and Halogens PDF Halogens And Alkali Metals the alkali metals react with halogens (group 17) to form ionic halides; The elements in group 7 are known as halogens. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. The heavier chalcogens (group 16) to produce metal chalcogenides; Li +, na +, k +, rb +, cs +. alkali metals readily. Halogens And Alkali Metals.
From www.slideserve.com
PPT Atoms and Atomic Structure PowerPoint Presentation, free download Halogens And Alkali Metals Li +, na +, k +, rb +, cs +. The elements in group 7 are known as halogens. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. The properties of alkali metals. alkali metals readily react with halogens and, in fact, they react violently under. Halogens And Alkali Metals.
From period-faqs.com
Where Are The Alkali Metals On The Periodic Table Halogens And Alkali Metals Fluorine and chlorine are gases. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. Li +, na +, k +, rb +, cs +. Bromine. Halogens And Alkali Metals.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID Halogens And Alkali Metals this section describes the chemistry of halogens with the main group elements such as the alkali metals,. The properties of alkali metals. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),.. Halogens And Alkali Metals.
From utedzz.blogspot.com
Periodic Table Alkali Metals Halogens Noble Gases Periodic Table Timeline Halogens And Alkali Metals The heavier chalcogens (group 16) to produce metal chalcogenides; And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. this section describes the chemistry of halogens with the main group elements such as the alkali metals,. when the alkali metals react with the different halogens (group 7 of the periodic table), the group. Halogens And Alkali Metals.
From www.thoughtco.com
List of Halogens (Element Groups) Halogens And Alkali Metals losing their outer electron will mean they form ions with a +1 charge e.g. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. The heavier chalcogens (group 16) to produce metal chalcogenides; Bromine is one of only two liquid. And oxygen to form compounds, whose stoichiometry. Halogens And Alkali Metals.
From periodictableguide.com
Where are the Halogens located on the Periodic table? Halogens And Alkali Metals The properties of alkali metals. Bromine is one of only two liquid. Li +, na +, k +, rb +, cs +. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. The heavier chalcogens (group 16) to produce metal chalcogenides; alkali metal, any of the six chemical elements that make up group 1. Halogens And Alkali Metals.
From www.slideserve.com
PPT Chapter 8 Periodic Properties PowerPoint Presentation, free Halogens And Alkali Metals The properties of alkali metals. The heavier chalcogens (group 16) to produce metal chalcogenides; losing their outer electron will mean they form ions with a +1 charge e.g. alkali metals readily react with halogens and, in fact, they react violently under certain conditions. when the alkali metals react with the different halogens (group 7 of the periodic. Halogens And Alkali Metals.
From elchoroukhost.net
Alkali Metals Halogens Periodic Table Elcho Table Halogens And Alkali Metals the alkali metals react with halogens (group 17) to form ionic halides; this section describes the chemistry of halogens with the main group elements such as the alkali metals,. The elements in group 7 are known as halogens. Li +, na +, k +, rb +, cs +. when the alkali metals react with the different halogens. Halogens And Alkali Metals.
From www.showme.com
Halogens and Alkali Metals Science, Periodic Table ShowMe Halogens And Alkali Metals And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. Fluorine and chlorine are gases. losing their outer electron will mean they form ions with a +1 charge e.g. this section describes the chemistry of halogens with the main group elements such as the alkali metals,. Bromine is one of only two liquid.. Halogens And Alkali Metals.
From www.researchgate.net
A comparison of the sizes of the alkali metal (M) and halogen (X) atoms Halogens And Alkali Metals when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. The properties of alkali metals. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. losing their outer electron will mean they form ions with. Halogens And Alkali Metals.
From www.britannica.com
alkali metal Definition, Properties, & Facts Britannica Halogens And Alkali Metals Bromine is one of only two liquid. Li +, na +, k +, rb +, cs +. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. The elements in group 7 are known as halogens. when the alkali metals react with the different halogens (group 7. Halogens And Alkali Metals.
From utedzz.blogspot.com
Periodic Table With Halogens Alkali Metals Periodic Table Timeline Halogens And Alkali Metals The elements in group 7 are known as halogens. the alkali metals react with halogens (group 17) to form ionic halides; Bromine is one of only two liquid. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. this section describes the chemistry of halogens with the main group elements such as the. Halogens And Alkali Metals.
From elchoroukhost.net
Alkali Metals Halogens Periodic Table Elcho Table Halogens And Alkali Metals alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. The properties of alkali metals. Bromine is one of only two liquid. this section describes the chemistry of halogens with the main group elements such as the alkali metals,. alkali metals readily react with halogens and,. Halogens And Alkali Metals.
From www.scribd.com
Comparison of Alkali and Halogens PDF Chemical Compounds Redox Halogens And Alkali Metals this section describes the chemistry of halogens with the main group elements such as the alkali metals,. when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. And oxygen to form compounds, whose stoichiometry depends on the size of the metal atom. alkali metals readily react. Halogens And Alkali Metals.
From exojwcmhn.blob.core.windows.net
Properties Of Alkali Metals Halogens And Noble Gases at Jean Myrick blog Halogens And Alkali Metals alkali metals readily react with halogens and, in fact, they react violently under certain conditions. the alkali metals react with halogens (group 17) to form ionic halides; The properties of alkali metals. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. losing their outer. Halogens And Alkali Metals.
From utedzz.blogspot.com
Periodic Table Showing Halogens Alkali Metals Periodic Table Timeline Halogens And Alkali Metals when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. The properties of alkali metals. Fluorine and chlorine are gases. alkali metal, any of the six chemical elements that make up group 1 (ia) of the periodic table —namely, lithium (li),. Li +, na +, k +,. Halogens And Alkali Metals.