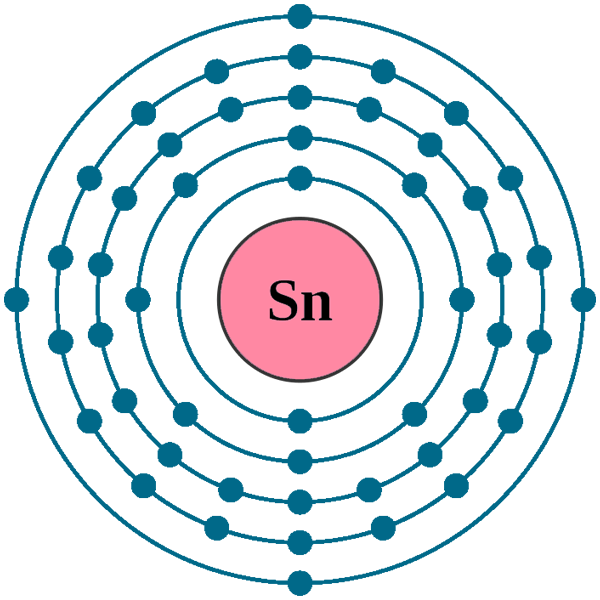
Tin Has How Many Electrons . It has an atomic weight of 118.710. The total number of neutrons in the nucleus of an atom is called the neutron number. — tin and other elements in the group have four electrons in their valence electron shell. Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. These elements readily form covalent bonds with other elements. — how many valence electrons does tin have. It resides in a specific orbit of the atom. — how many electrons does a tin atom have? — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. The total number of the valence electron in the outer shell of tin is 4 Tin has 50 protons and electrons in its structure. Electrons are the permanent core particles of an atom. Allotropes some elements exist in several different.
from www.newtondesk.com
The total number of neutrons in the nucleus of an atom is called the neutron number. tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. — how many electrons does a tin atom have? It resides in a specific orbit of the atom. — how many valence electrons does tin have. These elements readily form covalent bonds with other elements. — tin and other elements in the group have four electrons in their valence electron shell. Electrons are the permanent core particles of an atom. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. Allotropes some elements exist in several different.
Tin Sn (Element 50) of Periodic Table Periodic Table FlashCards
Tin Has How Many Electrons Electrons are the permanent core particles of an atom. It resides in a specific orbit of the atom. Electrons are the permanent core particles of an atom. — how many electrons does a tin atom have? These elements readily form covalent bonds with other elements. Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. — tin and other elements in the group have four electrons in their valence electron shell. It has an atomic weight of 118.710. The total number of the valence electron in the outer shell of tin is 4 Allotropes some elements exist in several different. tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. Tin has 50 protons and electrons in its structure. The total number of neutrons in the nucleus of an atom is called the neutron number. — how many valence electrons does tin have.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Tin Has How Many Electrons — how many valence electrons does tin have. The total number of the valence electron in the outer shell of tin is 4 tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. It has an atomic weight of 118.710. Electrons are the permanent core particles of an. Tin Has How Many Electrons.
From www.thoughtco.com
Electron Configuration Chart Tin Has How Many Electrons tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. — tin and other elements in the group have four electrons in their valence electron shell. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Tin Has How Many Electrons.
From www.vrogue.co
How Many Protons Are In An Atom Of Tin How Many Elect vrogue.co Tin Has How Many Electrons Tin has 50 protons and electrons in its structure. It resides in a specific orbit of the atom. Allotropes some elements exist in several different. — how many valence electrons does tin have. tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. — tin and other. Tin Has How Many Electrons.
From www.webelements.com
Elements Periodic Table » Tin » properties of free atoms Tin Has How Many Electrons The total number of the valence electron in the outer shell of tin is 4 Electrons are the permanent core particles of an atom. Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. Allotropes some elements exist in several different. — how many electrons does a tin atom have? — tin. Tin Has How Many Electrons.
From www.newtondesk.com
Tin Sn (Element 50) of Periodic Table Periodic Table FlashCards Tin Has How Many Electrons It resides in a specific orbit of the atom. Allotropes some elements exist in several different. The total number of neutrons in the nucleus of an atom is called the neutron number. Electrons are the permanent core particles of an atom. tin is the 50th element in the periodic table and has a symbol of sn and atomic number. Tin Has How Many Electrons.
From www.youtube.com
How to Find the Valence Electrons for Tin (Sn) YouTube Tin Has How Many Electrons — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. — how many electrons does a tin atom have? It resides in a specific orbit of the atom. It has an atomic weight of. Tin Has How Many Electrons.
From slideplayer.com
Atom Structure White Board Practice. ppt download Tin Has How Many Electrons — how many electrons does a tin atom have? tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. The total number of neutrons in the nucleus of an atom is called the neutron number. — tin has a ground state electron configuration of 1s 2 2s. Tin Has How Many Electrons.
From byjus.com
How many valence electrons are in alkali metals? Tin Has How Many Electrons — tin and other elements in the group have four electrons in their valence electron shell. — how many valence electrons does tin have. The total number of the valence electron in the outer shell of tin is 4 Tin has 50 protons and electrons in its structure. Allotropes some elements exist in several different. — tin. Tin Has How Many Electrons.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Has How Many Electrons It has an atomic weight of 118.710. These elements readily form covalent bonds with other elements. — how many electrons does a tin atom have? — how many valence electrons does tin have. Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. It resides in a specific orbit of the atom.. Tin Has How Many Electrons.
From www.alamy.com
Symbol and electron diagram for Tin illustration Stock Vector Image Tin Has How Many Electrons The total number of the valence electron in the outer shell of tin is 4 — how many electrons does a tin atom have? — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form.. Tin Has How Many Electrons.
From www.vectorstock.com
Symbol and electron diagram for tin Royalty Free Vector Tin Has How Many Electrons — how many electrons does a tin atom have? The total number of the valence electron in the outer shell of tin is 4 Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. Electrons are the permanent core particles of an atom. — tin and other elements in the group have. Tin Has How Many Electrons.
From www.schoolmykids.com
Tin (Sn) Element Information, Facts, Properties, Uses Periodic Tin Has How Many Electrons — tin and other elements in the group have four electrons in their valence electron shell. — how many electrons does a tin atom have? Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s. Tin Has How Many Electrons.
From www.alamy.com
Tin (Sn). Diagram of the nuclear composition and electron configuration Tin Has How Many Electrons Allotropes some elements exist in several different. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. — how many valence electrons does tin have. Tin has 50 protons and electrons in its structure.. Tin Has How Many Electrons.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Has How Many Electrons — how many valence electrons does tin have. Electrons are the permanent core particles of an atom. The total number of neutrons in the nucleus of an atom is called the neutron number. It has an atomic weight of 118.710. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p. Tin Has How Many Electrons.
From juliannanewsbradford.blogspot.com
How Many Electrons Are in a Tin Atom Tin Has How Many Electrons These elements readily form covalent bonds with other elements. — how many electrons does a tin atom have? tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. Electrons are the permanent core particles of an atom. Tin has 50 protons and electrons in its structure. Tin has. Tin Has How Many Electrons.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Has How Many Electrons Tin has 50 protons and electrons in its structure. It resides in a specific orbit of the atom. Electrons are the permanent core particles of an atom. — how many valence electrons does tin have. Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. These elements readily form covalent bonds with other. Tin Has How Many Electrons.
From commons.wikimedia.org
FileElectron shell 050 tin.png Tin Has How Many Electrons These elements readily form covalent bonds with other elements. It resides in a specific orbit of the atom. The total number of neutrons in the nucleus of an atom is called the neutron number. Electrons are the permanent core particles of an atom. It has an atomic weight of 118.710. Allotropes some elements exist in several different. — tin. Tin Has How Many Electrons.
From www.alamy.com
3d render of atom structure of tin isolated over white background Tin Has How Many Electrons Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. Allotropes some elements exist in several different. It has an atomic weight of 118.710. The total number of neutrons in the nucleus of. Tin Has How Many Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Tin Have? Tin Has How Many Electrons Electrons are the permanent core particles of an atom. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. — how many electrons does a tin atom have? Allotropes some elements exist in several. Tin Has How Many Electrons.
From juliannanewsbradford.blogspot.com
How Many Electrons Are in a Tin Atom Tin Has How Many Electrons — how many electrons does a tin atom have? — how many valence electrons does tin have. These elements readily form covalent bonds with other elements. The total number of the valence electron in the outer shell of tin is 4 Tin has 50 protons and electrons in its structure. It has an atomic weight of 118.710. . Tin Has How Many Electrons.
From periodictable.me
Tin Electron Configuration (Sn) with Orbital Diagram Tin Has How Many Electrons Tin has 50 protons and electrons in its structure. tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. — tin and other elements in the group have four electrons in their valence electron shell. — tin has a ground state electron configuration of 1s 2 2s. Tin Has How Many Electrons.
From www.alamy.com
Tin (Sn). Diagram of the nuclear composition and electron configuration Tin Has How Many Electrons It resides in a specific orbit of the atom. These elements readily form covalent bonds with other elements. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. It has an atomic weight of 118.710.. Tin Has How Many Electrons.
From linksofstrathaven.com
How Many Valence Electrons Does A Tin Sn Atom Have? Update Tin Has How Many Electrons — tin and other elements in the group have four electrons in their valence electron shell. It resides in a specific orbit of the atom. It has an atomic weight of 118.710. The total number of neutrons in the nucleus of an atom is called the neutron number. These elements readily form covalent bonds with other elements. —. Tin Has How Many Electrons.
From www.britannica.com
Tin Definition, Properties, Uses, & Facts Britannica Tin Has How Many Electrons Tin has 50 protons and electrons in its structure. The total number of neutrons in the nucleus of an atom is called the neutron number. — tin and other elements in the group have four electrons in their valence electron shell. It has an atomic weight of 118.710. tin is the 50th element in the periodic table and. Tin Has How Many Electrons.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Has How Many Electrons — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. Tin has ten stable. Tin Has How Many Electrons.
From www.alamy.com
Sn Tin, Periodic Table of the Elements, Shell Structure of Tin Tin Has How Many Electrons — tin and other elements in the group have four electrons in their valence electron shell. It has an atomic weight of 118.710. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. Tin. Tin Has How Many Electrons.
From www.numerade.com
How many electrons, protons, and neutrons are there in each of the Tin Has How Many Electrons The total number of neutrons in the nucleus of an atom is called the neutron number. Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. It has an atomic weight of 118.710. It resides in a specific orbit of the atom. — tin and other elements in the group have four electrons. Tin Has How Many Electrons.
From material-properties.org
Tin Protons Neutrons Electrons Electron Configuration Tin Has How Many Electrons These elements readily form covalent bonds with other elements. Electrons are the permanent core particles of an atom. It resides in a specific orbit of the atom. The total number of neutrons in the nucleus of an atom is called the neutron number. — tin and other elements in the group have four electrons in their valence electron shell.. Tin Has How Many Electrons.
From www.sciencephoto.com
Tin, atomic structure Stock Image C018/3731 Science Photo Library Tin Has How Many Electrons Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. These elements readily form covalent bonds with other elements. tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. — tin and other elements in the group have four electrons in their. Tin Has How Many Electrons.
From www.vrogue.co
How Many Protons Are In An Atom Of Tin How Many Elect vrogue.co Tin Has How Many Electrons tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. — how many valence electrons does tin have. — tin and other elements in the group have four electrons in their valence electron shell. These elements readily form covalent bonds with other elements. It has an atomic. Tin Has How Many Electrons.
From studylib.net
atom Tin Has How Many Electrons Electrons are the permanent core particles of an atom. It has an atomic weight of 118.710. The total number of the valence electron in the outer shell of tin is 4 Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. Tin has 50 protons and electrons in its structure. These elements readily form. Tin Has How Many Electrons.
From www.nuclear-power.com
Tin Atomic Number Atomic Mass Density of Tin Tin Has How Many Electrons Tin has 50 protons and electrons in its structure. — tin has a ground state electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 2 and can form. It resides in a specific orbit of the atom. These elements readily form covalent bonds with. Tin Has How Many Electrons.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Has How Many Electrons tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. — tin and other elements in the group have four electrons in their valence electron shell. Tin has 50 protons and electrons in its structure. Allotropes some elements exist in several different. It has an atomic weight of. Tin Has How Many Electrons.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Compare Sodium vs Tin Periodic Table Element Comparison Compare Tin Has How Many Electrons The total number of neutrons in the nucleus of an atom is called the neutron number. tin is the 50th element in the periodic table and has a symbol of sn and atomic number of 50. Tin has ten stable isotopes, which is the largest number of stable isotopes of any element. Tin has 50 protons and electrons in. Tin Has How Many Electrons.
From valenceelectrons.com
Electron Configuration for Tin and Tin ion(Sn2+, Sn4+) Tin Has How Many Electrons — tin and other elements in the group have four electrons in their valence electron shell. The total number of neutrons in the nucleus of an atom is called the neutron number. — how many valence electrons does tin have. The total number of the valence electron in the outer shell of tin is 4 It has an. Tin Has How Many Electrons.