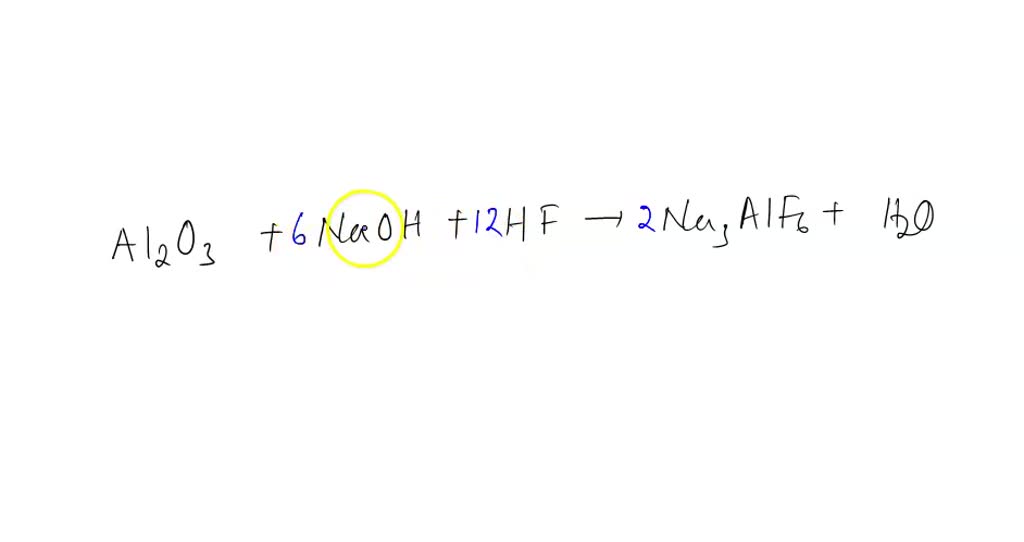Aluminum Oxide Balanced Formula . It shows the reactants (substances that start a reaction) and products (substances. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. But if the subscript 2 is added to make h2o2 h. A chemical equation represents a chemical reaction. How do you write the. Aluminum oxide = aluminium + dioxygen. If the coefficient 2 is added in front, that makes 2 water molecules; Original molecule h2o h 2 o: Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are.
from www.numerade.com
But if the subscript 2 is added to make h2o2 h. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. It shows the reactants (substances that start a reaction) and products (substances. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. How do you write the. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Original molecule h2o h 2 o: Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. A chemical equation represents a chemical reaction. Aluminum oxide = aluminium + dioxygen.
SOLVED Cryolile , Nay AIF6(S) , an Ore used in the production of aluminum; can be synthesized
Aluminum Oxide Balanced Formula It shows the reactants (substances that start a reaction) and products (substances. A chemical equation represents a chemical reaction. Original molecule h2o h 2 o: But if the subscript 2 is added to make h2o2 h. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. Aluminum oxide = aluminium + dioxygen. If the coefficient 2 is added in front, that makes 2 water molecules; How do you write the. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. It shows the reactants (substances that start a reaction) and products (substances.
From www.myxxgirl.com
Aluminium Oxide Formula How To Write The Formula My XXX Hot Girl Aluminum Oxide Balanced Formula Original molecule h2o h 2 o: Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. It shows the reactants (substances that start a reaction) and products (substances. Aluminum oxide = aluminium + dioxygen. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Aluminum Oxide Balanced Formula.
From www.youtube.com
Writing the Formula for Aluminum Oxide YouTube Aluminum Oxide Balanced Formula It shows the reactants (substances that start a reaction) and products (substances. A chemical equation represents a chemical reaction. But if the subscript 2 is added to make h2o2 h. Original molecule h2o h 2 o: The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. To. Aluminum Oxide Balanced Formula.
From testbook.com
Aluminium Oxide Formula Concept, Structure, Properties and Uses. Aluminum Oxide Balanced Formula Original molecule h2o h 2 o: Aluminum oxide = aluminium + dioxygen. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. A chemical equation represents a chemical reaction. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. How. Aluminum Oxide Balanced Formula.
From www.numerade.com
SOLVED Aluminum reacts with oxygen to produce aluminum oxide based on the balanced equation 4 Aluminum Oxide Balanced Formula Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. It shows the reactants (substances that start a reaction) and products (substances. If the coefficient 2 is added in front, that makes 2 water molecules; Aluminum oxide = aluminium +. Aluminum Oxide Balanced Formula.
From www.youtube.com
Al+Fe2O3=Al2O3+Fe Balanced EquationAluminum+Iron(III) oxide+Iron Balanced Equation YouTube Aluminum Oxide Balanced Formula Original molecule h2o h 2 o: Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances. If the coefficient 2 is. Aluminum Oxide Balanced Formula.
From eurassignmentcot.web.fc2.com
How to write aluminum oxide as a formula Aluminum Oxide Balanced Formula Original molecule h2o h 2 o: It shows the reactants (substances that start a reaction) and products (substances. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Aluminum oxide = aluminium + dioxygen. But if the subscript 2 is added to make h2o2 h. If the coefficient 2 is added in front, that makes. Aluminum Oxide Balanced Formula.
From www.pw.live
Aluminium Oxide Formula, Structure, Properties, Uses Aluminum Oxide Balanced Formula The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Aluminum oxide = aluminium + dioxygen. How do you write the. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. If the coefficient 2 is added in front, that makes 2 water molecules;. Aluminum Oxide Balanced Formula.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free download ID4439360 Aluminum Oxide Balanced Formula A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances. Aluminum oxide = aluminium + dioxygen. If the coefficient 2 is added in front, that makes 2 water molecules; The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction. Aluminum Oxide Balanced Formula.
From www.numerade.com
SOLVED Write the balanced equation for the formation of aluminum oxide from its elements. How Aluminum Oxide Balanced Formula Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. If the coefficient 2 is added in front, that makes 2 water molecules; Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Original molecule h2o h 2 o: It shows the reactants (substances that start a reaction) and products (substances. The. Aluminum Oxide Balanced Formula.
From www.numerade.com
SOLVEDAluminum oxidizes to form aluminum oxide. a. Write the balanced chemical equation for the Aluminum Oxide Balanced Formula If the coefficient 2 is added in front, that makes 2 water molecules; Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. It shows the reactants (substances that start a reaction) and products (substances. How do you write the. Original molecule h2o h 2 o: A chemical equation represents a chemical reaction. But if the subscript. Aluminum Oxide Balanced Formula.
From bernard-blogrose.blogspot.com
Aluminum Reacts With Oxygen to Produce Aluminum Oxide Chemical Equation Aluminum Oxide Balanced Formula It shows the reactants (substances that start a reaction) and products (substances. If the coefficient 2 is added in front, that makes 2 water molecules; Original molecule h2o h 2 o: Aluminum oxide = aluminium + dioxygen. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are.. Aluminum Oxide Balanced Formula.
From www.youtube.com
Al2O3+H2SO4=Al2(SO4)3+H2O Balanced EquationAluminum oxide+Sulphuric acid=Aluminum sulphate Aluminum Oxide Balanced Formula Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Original molecule h2o h 2 o: A chemical equation represents a chemical reaction. But if the subscript 2 is added to make h2o2 h. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. Aluminum oxide = aluminium + dioxygen. How do. Aluminum Oxide Balanced Formula.
From www.meritnation.com
Ferric oxide reacts with aluminium to produce aluminium oxide and iron The balanced chemical Aluminum Oxide Balanced Formula Original molecule h2o h 2 o: If the coefficient 2 is added in front, that makes 2 water molecules; Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A chemical equation represents a chemical reaction. Aluminum sulfate,. Aluminum Oxide Balanced Formula.
From www.youtube.com
Write a balanced equation for the reaction betwee aluminium oxide & sodium hydroxide solution Aluminum Oxide Balanced Formula How do you write the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. If the coefficient 2 is added in front, that makes 2 water molecules; A chemical equation represents a chemical reaction. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Original molecule h2o. Aluminum Oxide Balanced Formula.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas Aluminum Oxide Balanced Formula To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. If the coefficient 2 is added in front, that makes 2 water molecules; Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. But if the subscript 2 is added to make h2o2 h. How do you write. Aluminum Oxide Balanced Formula.
From questions.kunduz.com
When iron(III) oxide reacts with aluminum... Physical Chemistry Aluminum Oxide Balanced Formula A chemical equation represents a chemical reaction. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. How do you write the. But if the subscript 2 is added to make h2o2 h. To balance a chemical equation, enter an equation of a chemical reaction and press. Aluminum Oxide Balanced Formula.
From www.numerade.com
SOLVED Cryolile , Nay AIF6(S) , an Ore used in the production of aluminum; can be synthesized Aluminum Oxide Balanced Formula It shows the reactants (substances that start a reaction) and products (substances. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. But if the subscript 2 is added to make h2o2 h. How do you write the. If the coefficient 2 is added in front, that makes 2 water molecules; Al2o3 = al + o2 is. Aluminum Oxide Balanced Formula.
From bernard-blogrose.blogspot.com
Aluminum Reacts With Oxygen to Produce Aluminum Oxide Chemical Equation Aluminum Oxide Balanced Formula A chemical equation represents a chemical reaction. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Aluminum Oxide Balanced Formula.
From www.museoinclusivo.com
Exploring the Chemical Formula of Aluminum Oxide Aluminum Profile Blog Aluminum Oxide Balanced Formula Aluminum oxide = aluminium + dioxygen. But if the subscript 2 is added to make h2o2 h. Original molecule h2o h 2 o: How do you write the. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. A chemical equation represents a chemical reaction. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and. Aluminum Oxide Balanced Formula.
From www.chegg.com
Solved Aluminum Metal Reacts With Oxygen Gas To Form Alum... Aluminum Oxide Balanced Formula Original molecule h2o h 2 o: It shows the reactants (substances that start a reaction) and products (substances. How do you write the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in. Aluminum Oxide Balanced Formula.
From www.numerade.com
SOLVED Aluminum metal reacts with oxygen gas to produce aluminum oxide solid. Write the Aluminum Oxide Balanced Formula It shows the reactants (substances that start a reaction) and products (substances. How do you write the. Aluminum oxide = aluminium + dioxygen. If the coefficient 2 is added in front, that makes 2 water molecules; To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Original molecule h2o h 2 o: A. Aluminum Oxide Balanced Formula.
From www.youtube.com
Write the chemical formula of Aluminium oxide YouTube Aluminum Oxide Balanced Formula Original molecule h2o h 2 o: Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. How do you write the. But if the subscript 2 is added to make h2o2 h. Aluminum oxide = aluminium + dioxygen. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. A chemical equation represents. Aluminum Oxide Balanced Formula.
From www.numerade.com
SOLVED Question 2 10 pts In a redox reaction, solid iron (III) oxide and powdered aluminum Aluminum Oxide Balanced Formula But if the subscript 2 is added to make h2o2 h. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. If the coefficient 2 is added in front, that makes 2 water molecules; Original molecule h2o h 2 o: Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. To balance. Aluminum Oxide Balanced Formula.
From www.meritnation.com
Write balanced chemical equation for Aluminium oxide and sodium oxide Science Chemical Aluminum Oxide Balanced Formula To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. It shows the reactants (substances that start a reaction) and products (substances. But if the subscript 2 is added to make h2o2 h. How do you write the. Aluminum oxide = aluminium + dioxygen. Original molecule h2o h 2 o: A chemical equation. Aluminum Oxide Balanced Formula.
From www.youtube.com
Aluminium Oxide Balanced symbol equation YouTube Aluminum Oxide Balanced Formula A chemical equation represents a chemical reaction. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. If the coefficient 2 is added in front, that makes 2 water molecules; Original molecule h2o h 2 o: Al2o3 = al + o2 is a decomposition reaction where two. Aluminum Oxide Balanced Formula.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Balanced Equation For Aluminum And Oxygen Gas Aluminum Oxide Balanced Formula Aluminum oxide = aluminium + dioxygen. But if the subscript 2 is added to make h2o2 h. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. If the coefficient 2 is added in front, that makes 2 water molecules; Original molecule h2o h 2 o: Al2o3 = al + o2 is a. Aluminum Oxide Balanced Formula.
From www.chegg.com
Solved Aluminum reacts with oxygen to produce aluminum oxide Aluminum Oxide Balanced Formula A chemical equation represents a chemical reaction. If the coefficient 2 is added in front, that makes 2 water molecules; It shows the reactants (substances that start a reaction) and products (substances. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Original molecule h2o h 2 o: How do you write the. Aluminum sulfate,. Aluminum Oxide Balanced Formula.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Aluminum Oxide Balanced Formula Aluminum oxide = aluminium + dioxygen. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. A chemical equation represents a chemical reaction. How do you write the. But if the subscript 2 is added to make h2o2 h. Al2o3 = al + o2 is a decomposition. Aluminum Oxide Balanced Formula.
From www.museoinclusivo.com
Exploring the Formula for Aluminum Oxide Aluminum Profile Blog Aluminum Oxide Balanced Formula The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. A chemical equation represents a chemical reaction. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. Original molecule. Aluminum Oxide Balanced Formula.
From www.numerade.com
SOLVEDWrite a balanced equation to represent the electrolysis of aluminum oxide. If 2.00 L of Aluminum Oxide Balanced Formula Aluminum oxide = aluminium + dioxygen. Original molecule h2o h 2 o: It shows the reactants (substances that start a reaction) and products (substances. But if the subscript 2 is added to make h2o2 h. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. The chemical equation described in section 4.1 is balanced, meaning. Aluminum Oxide Balanced Formula.
From www.numerade.com
SOLVED a chemist burns 16.0 g of al in excess air to produce aluminum oxide, Al2O3. She Aluminum Oxide Balanced Formula Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. But if the subscript 2 is added to make h2o2 h. A chemical equation represents a chemical reaction. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. If the coefficient 2 is added in front, that makes 2 water molecules; Aluminum. Aluminum Oxide Balanced Formula.
From www.numerade.com
SOLVED Aluminum sulfate, Al2(SO4)3, to form aluminum oxide, Al2O3, and sulfur Aluminum Oxide Balanced Formula But if the subscript 2 is added to make h2o2 h. If the coefficient 2 is added in front, that makes 2 water molecules; A chemical equation represents a chemical reaction. Al2o3 = al + o2 is a decomposition reaction where two moles of aluminum oxide. To balance a chemical equation, enter an equation of a chemical reaction and press. Aluminum Oxide Balanced Formula.
From www.youtube.com
How to Balance Al + O2 = Al2O3 YouTube Aluminum Oxide Balanced Formula Aluminum oxide = aluminium + dioxygen. Original molecule h2o h 2 o: How do you write the. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. A chemical equation represents a chemical reaction. It shows the reactants (substances that start a reaction) and products (substances. If. Aluminum Oxide Balanced Formula.
From brainly.in
3. Write balanced chemical equation.When Aluminium reacts with oxygen form its oxide. Why Aluminum Oxide Balanced Formula Original molecule h2o h 2 o: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. If the coefficient 2 is added in front, that makes 2 water molecules; Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. But if the subscript 2 is added to make h2o2 h.. Aluminum Oxide Balanced Formula.
From www.slideserve.com
PPT Word equations to balanced chemical equations drill Each of the following slides first Aluminum Oxide Balanced Formula To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A chemical equation represents a chemical reaction. Aluminum sulfate, #al_2(so_4)_3#, decomposes to form aluminum oxide, #al_2o_3#, and sulfur trioxide, #so_3#. How do you write the. If the coefficient 2 is added in front, that makes 2 water molecules; The chemical equation described in. Aluminum Oxide Balanced Formula.