Own Brand Labeling Medical Device . own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. 4.5/5 (544) the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases.
from www.drugwatch.com
own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. 4.5/5 (544) following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own.
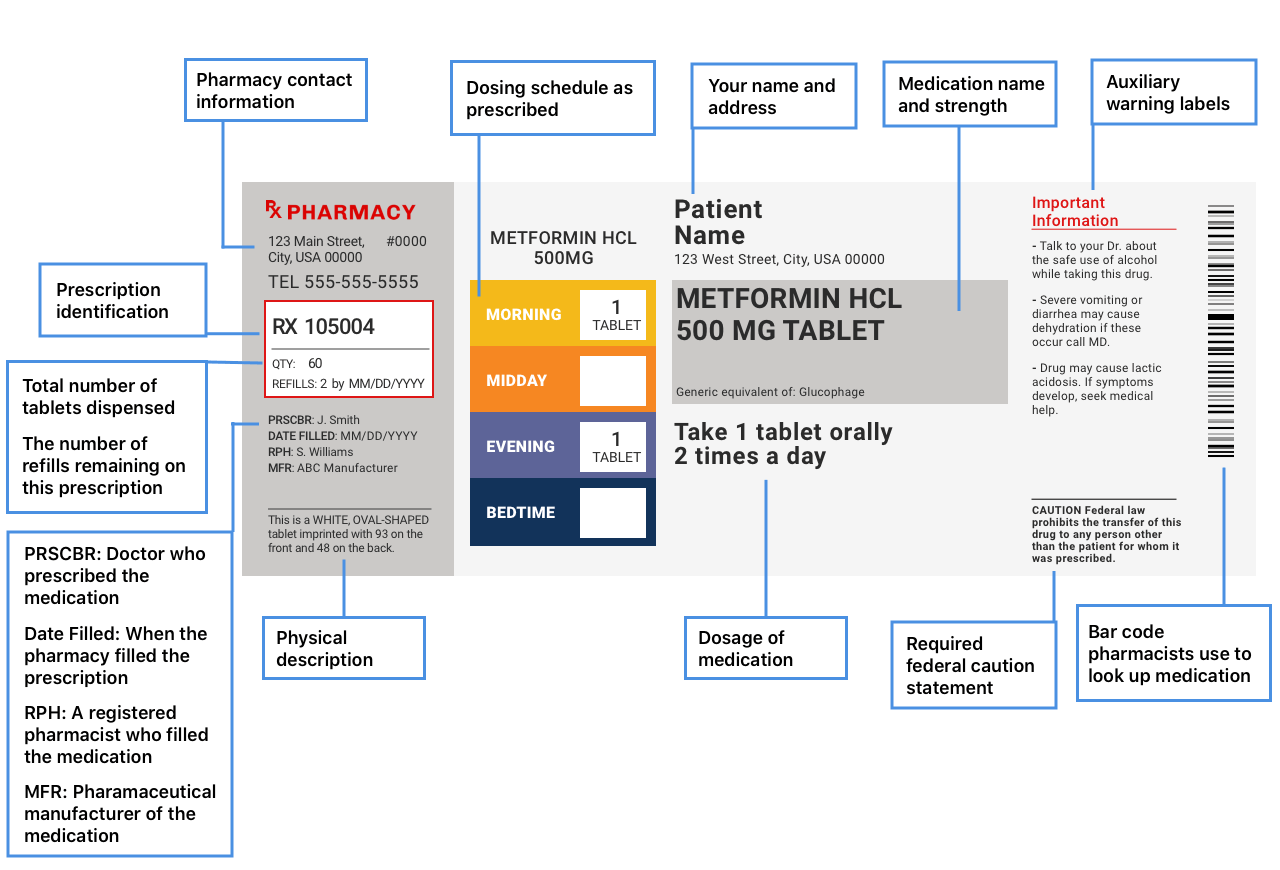
How to Read OvertheCounter and Prescription Drug Labels
Own Brand Labeling Medical Device own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. 4.5/5 (544) the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. guidance for manufacturers who don’t design or manufacture devices but place their names on the product.
From easymedicaldevice.com
OBL Own Brand Labelling Medical Devices (MDR 2017/745) Own Brand Labeling Medical Device 4.5/5 (544) guidance for manufacturers who don’t design or manufacture devices but place their names on the product. own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer. Own Brand Labeling Medical Device.
From www.labellingtopack.com
Understanding Product Labels What Is A Label? Own Brand Labeling Medical Device the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. 4.5/5 (544) own brand labelling or obl is the. Own Brand Labeling Medical Device.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Own Brand Labeling Medical Device following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. the specific situation which is usually described as “own. Own Brand Labeling Medical Device.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Own Brand Labeling Medical Device following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. own brand labelling. Own Brand Labeling Medical Device.
From www.youtube.com
Own Brand Label 🏷️ Launch Drifit Manufacturer Chennai Wholesale Own Brand Labeling Medical Device following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. 4.5/5 (544) own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. the regulatory changes introduced by. Own Brand Labeling Medical Device.
From ambitiousmares.blogspot.com
35 Medical Device Label Labels Design Ideas 2020 Own Brand Labeling Medical Device own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. 4.5/5 (544) the specific situation which is usually described. Own Brand Labeling Medical Device.
From www.pinterest.com
品牌|被这些医药品牌设计治愈了! Graphic Design Branding, Label Design, Package Design Own Brand Labeling Medical Device own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. 4.5/5 (544) own brand labelling. Own Brand Labeling Medical Device.
From www.youtube.com
How to read a medication label YouTube Own Brand Labeling Medical Device the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. own brand labelling. Own Brand Labeling Medical Device.
From www.designcrowd.com
Playful, Modern, Health Product Label Design for a Company by RenCan Own Brand Labeling Medical Device the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. 4.5/5 (544) following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. own brand. Own Brand Labeling Medical Device.
From clin-r.com
Labels for Medical Devices Clin R Own Brand Labeling Medical Device the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. 4.5/5 (544). Own Brand Labeling Medical Device.
From www.barcode-us.com
Medical Devices UDI Own Brand Labeling Medical Device own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. 4.5/5 (544) . Own Brand Labeling Medical Device.
From mediqueproducts.com
Medique Products The Brands That Work Own Brand Labeling Medical Device guidance for manufacturers who don’t design or manufacture devices but place their names on the product. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. own brand labelling or obl is the action to buy. Own Brand Labeling Medical Device.
From www.inkablelabel.com
A StepbyStep Guide to Designing and Creating Your Product Label Own Brand Labeling Medical Device own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. 4.5/5 . Own Brand Labeling Medical Device.
From www.printrunner.com
How to Make an Effective Product Label Design PrintRunner Blog Own Brand Labeling Medical Device following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. 4.5/5 (544) own brand labelling or obl is the action to buy a product that is already finished from another manufacturer. Own Brand Labeling Medical Device.
From blog.durafastlabel.com
Building Your Brand By Printing Your Own Color Labels DuraFast Label Own Brand Labeling Medical Device the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. 4.5/5 (544) the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. own brand labelling or. Own Brand Labeling Medical Device.
From nameplatesforindustry.com
Medical Equipment Labels NFI Corp Nameplates for Industry Own Brand Labeling Medical Device guidance for manufacturers who don’t design or manufacture devices but place their names on the product. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. following the ec recommendation. Own Brand Labeling Medical Device.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Own Brand Labeling Medical Device the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. own brand labelling or obl is the action to buy. Own Brand Labeling Medical Device.
From www.pinterest.com.mx
Startup Medical Device company needs sharp new logo! by May Gupita Own Brand Labeling Medical Device guidance for manufacturers who don’t design or manufacture devices but place their names on the product. 4.5/5 (544) own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. the specific situation which is usually described. Own Brand Labeling Medical Device.
From oem-cosmetic.com
OEM/White Label What It Is And How It Works 株式会社OEM Own Brand Labeling Medical Device the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. 4.5/5 (544) the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. own brand labelling (obl). Own Brand Labeling Medical Device.
From www.pharmavisualaid.in
Importance of Labeling in the Pharmaceutical industry Pharma Visual Aid Own Brand Labeling Medical Device the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. 4.5/5 (544) following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. own brand labelling. Own Brand Labeling Medical Device.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint Own Brand Labeling Medical Device following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. 4.5/5 (544) own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. . Own Brand Labeling Medical Device.
From ambitiousmares.blogspot.com
31 What Is Product Label Labels Design Ideas 2020 Own Brand Labeling Medical Device own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. own brand labelling (obl). Own Brand Labeling Medical Device.
From www.prp-us.com
Major Regulatory Changes for OwnBrand Label (OBL) Manufacturers Own Brand Labeling Medical Device guidance for manufacturers who don’t design or manufacture devices but place their names on the product. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. following the ec recommendation 2013/473/eu, the medicines. Own Brand Labeling Medical Device.
From www.brandly.com.my
Brandly Build Your Own Brand Own Brand Labeling Medical Device own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. 4.5/5 . Own Brand Labeling Medical Device.
From www.prp-us.com
Major Regulatory Changes for OwnBrand Label (OBL) Manufacturers Own Brand Labeling Medical Device own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. 4.5/5 (544) own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases.. Own Brand Labeling Medical Device.
From www.zigpac.com
Private Labeling Methods of Branding Through 11 Solutions Zigpac Own Brand Labeling Medical Device the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. 4.5/5 (544) own brand labelling. Own Brand Labeling Medical Device.
From www.upmraflatac.com
UPM Raflatac introduces new labeling solutions for medical device and Own Brand Labeling Medical Device own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. 4.5/5 (544) own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry.. Own Brand Labeling Medical Device.
From paragondsi.com
UDI Unique Device Identification for Single and Multiple Uses Own Brand Labeling Medical Device the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. 4.5/5 (544) own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. the regulatory changes. Own Brand Labeling Medical Device.
From digitalstickers.com.au
Brand Labels Own Brand Labeling Medical Device the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. guidance for manufacturers who don’t design or manufacture devices. Own Brand Labeling Medical Device.
From www.thema-med.com
Own brand label manufacturing what's next? Thema Med Own Brand Labeling Medical Device own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. the regulatory changes introduced by the european regulations. Own Brand Labeling Medical Device.
From www.thema-med.com
Own brand label manufacturing what's next? Thema Med Own Brand Labeling Medical Device own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. guidance for manufacturers who don’t design. Own Brand Labeling Medical Device.
From adenelipackaging.com
Self Adhesive Labeling Machines Adeneli Packaging Own Brand Labeling Medical Device own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. following the ec recommendation 2013/473/eu,. Own Brand Labeling Medical Device.
From www.fda.gov
OTC Drug Facts Label FDA Own Brand Labeling Medical Device the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. the specific situation which is usually described as “own brand labelling” (obl), is where the legal manufacturer purchases. following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. own brand labelling or obl is. Own Brand Labeling Medical Device.
From easymedicaldevice.com
OBL Own Brand Labelling Medical Devices (MDR 2017/745) Own Brand Labeling Medical Device guidance for manufacturers who don’t design or manufacture devices but place their names on the product. own brand labelling or obl is the action to buy a product that is already finished from another manufacturer and to. 4.5/5 (544) own brand labelling (obl) represents a strategic opportunity for companies in the medical device industry. the. Own Brand Labeling Medical Device.
From blog.sierralabs.com
5 Best Practices for Pharmaceutical Labeling Own Brand Labeling Medical Device following the ec recommendation 2013/473/eu, the medicines and healthcare products regulatory agency (mhra) replaced the term “own. guidance for manufacturers who don’t design or manufacture devices but place their names on the product. the regulatory changes introduced by the european regulations mdr (eu) 2017/745 and the new obl. the specific situation which is usually described as. Own Brand Labeling Medical Device.