Magnesium Carbonate In Water Equation . Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one.
from www.numerade.com
The balanced equation will be calculated along with the. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. Enter an equation of an ionic chemical equation and press the balance button.
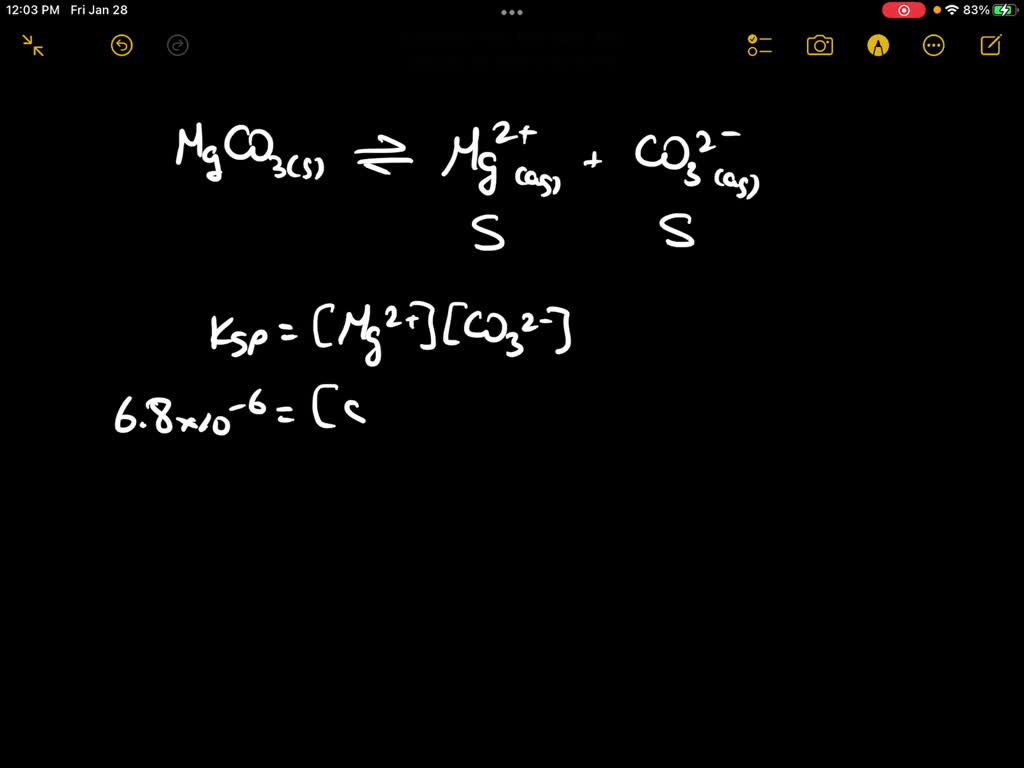
SOLVED The molar solubility of magnesium carbonate in a water solution
Magnesium Carbonate In Water Equation Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the.
From www.youtube.com
How to Balance MgCO3 = MgO + CO2 of Magnesium carbonate Magnesium Carbonate In Water Equation Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. The balanced equation will be calculated along with the. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide,. Magnesium Carbonate In Water Equation.
From ar.inspiredpencil.com
Magnesium And Water Reaction Magnesium Carbonate In Water Equation The balanced equation will be calculated along with the. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride. Magnesium Carbonate In Water Equation.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium Carbonate In Water Equation When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. When aqueous hydrochloric acid (hcl) is mixed. Magnesium Carbonate In Water Equation.
From www.pinterest.com
Making magnesium carbonate the formation of an insoluble salt in water Magnesium Carbonate In Water Equation When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and. Magnesium Carbonate In Water Equation.
From www.youtube.com
Equation for Magnesium Hydroxide Dissolving in Water Mg(OH)2 + H2O Magnesium Carbonate In Water Equation One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Enter an equation of an ionic chemical equation and press the balance button. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. The balanced equation will. Magnesium Carbonate In Water Equation.
From solvedlib.com
Geologists identify carbonate minerals by reaction wi… SolvedLib Magnesium Carbonate In Water Equation One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will. Magnesium Carbonate In Water Equation.
From bmp-brah.blogspot.com
Mgco3 Balanced Equation bmpbrah Magnesium Carbonate In Water Equation When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The balanced equation will be calculated along with the. Enter an equation of an ionic. Magnesium Carbonate In Water Equation.
From www.shutterstock.com
23 Magnesium Carbonate Formula Images, Stock Photos & Vectors Magnesium Carbonate In Water Equation The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate. Magnesium Carbonate In Water Equation.
From socratic.org
What is the name of MgCO_3? Socratic Magnesium Carbonate In Water Equation Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The balanced equation will be calculated along with the. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the. Magnesium Carbonate In Water Equation.
From www.dreamstime.com
Magnesium Carbonate Molecule Stock Vector Illustration of Magnesium Carbonate In Water Equation The balanced equation will be calculated along with the. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous. Magnesium Carbonate In Water Equation.
From smartfishingtips.com
Magnesium Carbonate What Is It And How Does It Effect The Magnesium Carbonate In Water Equation When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Enter an equation of an ionic chemical equation and press the balance button. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one.. Magnesium Carbonate In Water Equation.
From www.showme.com
ShowMe Magnesium carbonate Magnesium Carbonate In Water Equation Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. The balanced equation will be calculated along with the. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide,. Magnesium Carbonate In Water Equation.
From ar.inspiredpencil.com
Magnesium And Water Reaction Magnesium Carbonate In Water Equation One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will. Magnesium Carbonate In Water Equation.
From www.numerade.com
SOLVED The molar solubility of magnesium carbonate in a water solution Magnesium Carbonate In Water Equation In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Enter an equation of an ionic chemical equation and press the balance button. One mole of magnesium carbonate [mgco 3]. Magnesium Carbonate In Water Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation for the following Magnesium Carbonate In Water Equation When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. The balanced equation will be calculated along with the.. Magnesium Carbonate In Water Equation.
From www.slideserve.com
PPT Carbonate System Alkalinity PowerPoint Presentation, free Magnesium Carbonate In Water Equation In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. The balanced equation will be calculated along. Magnesium Carbonate In Water Equation.
From yesdirt.com
Is Magnesium Carbonate Soluble in Water? (Answered) Yes Dirt Magnesium Carbonate In Water Equation The balanced equation will be calculated along with the. Enter an equation of an ionic chemical equation and press the balance button. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. One mole of magnesium carbonate [mgco 3]. Magnesium Carbonate In Water Equation.
From dayanara-blogmosley.blogspot.com
Magnesium Sulfate and Sodium Carbonate Balanced Equation Magnesium Carbonate In Water Equation One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid.. Magnesium Carbonate In Water Equation.
From testbook.com
Magnesium Carbonate Learn Definition, Structure, Formula & Uses Magnesium Carbonate In Water Equation Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. Enter an. Magnesium Carbonate In Water Equation.
From delantalesybanderines.blogspot.com
Mgco3+Hcl=Mgcl2+H2O+Co2 Ionic Equation delantalesybanderines Magnesium Carbonate In Water Equation The balanced equation will be calculated along with the. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. Enter an equation of an ionic chemical equation and press the balance button. When aqueous hydrochloric acid (hcl) is mixed. Magnesium Carbonate In Water Equation.
From www.glentham.com
Magnesium carbonate, basic, chemical pure (CAS 39409820) Glentham Magnesium Carbonate In Water Equation The balanced equation will be calculated along with the. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. When carbon dioxide is dissolved in. Magnesium Carbonate In Water Equation.
From childhealthpolicy.vumc.org
⛔ Chemical reaction of magnesium and oxygen. What is the chemical Magnesium Carbonate In Water Equation Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen. Magnesium Carbonate In Water Equation.
From www.numerade.com
SOLVED Sadiq repeated the experiment by adding sulphuric acid to Magnesium Carbonate In Water Equation One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. The balanced equation will. Magnesium Carbonate In Water Equation.
From joildreqq.blob.core.windows.net
Magnesium Bicarbonate + Hydrochloric Acid at Gregory Sanders blog Magnesium Carbonate In Water Equation When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water.. Magnesium Carbonate In Water Equation.
From www.numerade.com
SOLVED C2. Magnesium carbonate reacts with phosphoric acid (H3PO4) to Magnesium Carbonate In Water Equation When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one.. Magnesium Carbonate In Water Equation.
From www.youtube.com
Why does Magnesium react with Water? YouTube Magnesium Carbonate In Water Equation When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts. Magnesium Carbonate In Water Equation.
From www.numerade.com
SOLVED A 15.9 g15.9 g sample of a mixture of magnesium carbonate and Magnesium Carbonate In Water Equation When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. Enter an equation of an ionic chemical. Magnesium Carbonate In Water Equation.
From www.chegg.com
Solved The Of Magnesium Carbonate Is Shown Magnesium Carbonate In Water Equation One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. The balanced equation will be calculated along with the. In this video we. Magnesium Carbonate In Water Equation.
From exoxihnad.blob.core.windows.net
Magnesium Carbonate Balanced Equation at Joseph Patton blog Magnesium Carbonate In Water Equation Enter an equation of an ionic chemical equation and press the balance button. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid. The balanced equation will be calculated along with the. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide,. Magnesium Carbonate In Water Equation.
From www.numerade.com
SOLVED Magnesium carbonate, magnesium oxide, and magnesium hydroxide Magnesium Carbonate In Water Equation The balanced equation will be calculated along with the. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Magnesium carbonate + phosphoric acid =. Magnesium Carbonate In Water Equation.
From www.dreamstime.com
3D Image of Magnesium Carbonate Skeletal Formula Stock Illustration Magnesium Carbonate In Water Equation When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. Enter an. Magnesium Carbonate In Water Equation.
From www.numerade.com
SOLVED A geologist discovers a magnesium carbonate hydroxide hydrated Magnesium Carbonate In Water Equation Enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along with the. In this video we will describe the equation mgco3 + h2o and write what happens when mgco3 is. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. When carbon dioxide is dissolved in an. Magnesium Carbonate In Water Equation.
From www.chegg.com
Solved of magnesium carbonate produces Magnesium Carbonate In Water Equation One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will give carbon dioxide, magnesium chloride and water. The balanced equation will be calculated along with the. Magnesium carbonate + phosphoric acid =. Magnesium Carbonate In Water Equation.
From www.numerade.com
SOLVED 2. Magnesium carbonate and sulfuric acid react to yield Magnesium Carbonate In Water Equation Enter an equation of an ionic chemical equation and press the balance button. Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. When aqueous hydrochloric acid (hcl) is mixed with solid. Magnesium Carbonate In Water Equation.
From www.youtube.com
The molar solubility of magnesium carbonate is 1.87x10^4 mol/L Magnesium Carbonate In Water Equation Magnesium carbonate + phosphoric acid = water + magnesium phosphate + carbon dioxide. One mole of magnesium carbonate [mgco 3] and two moles of hydrogen chloride [hcl] react to form one mole of magnesium chloride [mgcl 2], one. The balanced equation will be calculated along with the. When aqueous hydrochloric acid (hcl) is mixed with solid magnesium carbonate, it will. Magnesium Carbonate In Water Equation.