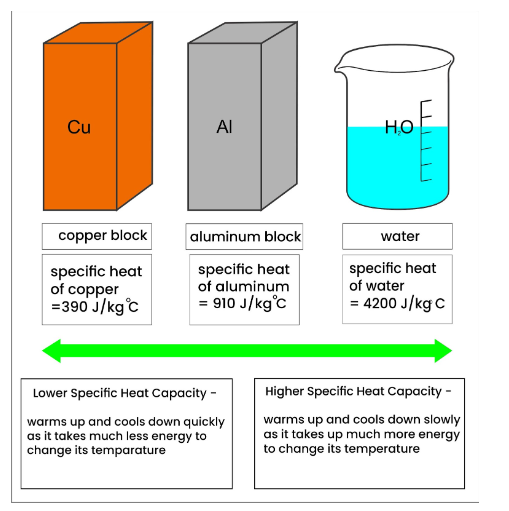
Why Does Lead Have A High Specific Heat Capacity . This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Specific heat of lead is 0.13 j/g k. Define heat capacity and specific heat capacity and differentiate between the two terms ; 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat, or specific heat capacity, is a property related to internal. Deduce which substance will have greatest. Water has a particularly high specific heat compared to many other substances.
from studymind.co.uk
Specific heat, or specific heat capacity, is a property related to internal. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Specific heat of lead is 0.13 j/g k. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Water has a particularly high specific heat compared to many other substances. Deduce which substance will have greatest. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Define heat capacity and specific heat capacity and differentiate between the two terms ;
Heat Capacity Study Mind
Why Does Lead Have A High Specific Heat Capacity Specific heat of lead is 0.13 j/g k. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Specific heat, or specific heat capacity, is a property related to internal. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Define heat capacity and specific heat capacity and differentiate between the two terms ; Deduce which substance will have greatest. Specific heat of lead is 0.13 j/g k. Water has a particularly high specific heat compared to many other substances.
From otrabalhosocomecou.macae.rj.gov.br
Übertragung Kategorie Experiment heat capacity of wood Strand Mit anderen Bands Start Why Does Lead Have A High Specific Heat Capacity Water has a particularly high specific heat compared to many other substances. Specific heat of lead is 0.13 j/g k. Deduce which substance will have greatest. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by. Why Does Lead Have A High Specific Heat Capacity.
From www.animalia-life.club
Specific Heat Chart Of Common Substances Why Does Lead Have A High Specific Heat Capacity Specific heat of lead is 0.13 j/g k. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Specific heat, or specific heat capacity, is a property related to internal. Define heat capacity and specific heat capacity and differentiate between the two terms. Why Does Lead Have A High Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID1596737 Why Does Lead Have A High Specific Heat Capacity Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Deduce which substance will have greatest. Specific heat, or specific heat capacity, is a property related to internal. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. This explanation explains why the. Why Does Lead Have A High Specific Heat Capacity.
From www.youtube.com
Determining the Specific Heat Capacity of Materials GCSE Physics YouTube Why Does Lead Have A High Specific Heat Capacity Specific heat, or specific heat capacity, is a property related to internal. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Deduce which substance will have greatest. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Specific. Why Does Lead Have A High Specific Heat Capacity.
From www.downwindkennels.com
Specific Heat Capacity Why Does Lead Have A High Specific Heat Capacity 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Its specific heat capacity is 4.184 j/g°c, which means it. Why Does Lead Have A High Specific Heat Capacity.
From lessonmagicziegler.z21.web.core.windows.net
Worksheet Introduction To Specific Heat Capacities Why Does Lead Have A High Specific Heat Capacity Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Water has a particularly high specific heat compared to many other substances. Specific heat, or specific heat capacity, is a property related to internal. Specific heat of lead is 0.13 j/g k. Define heat capacity and specific heat capacity and differentiate between the two terms ;. Why Does Lead Have A High Specific Heat Capacity.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Why Does Lead Have A High Specific Heat Capacity Specific heat, or specific heat capacity, is a property related to internal. Water has a particularly high specific heat compared to many other substances. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Deduce which substance will have greatest. Define heat capacity and specific heat capacity. Why Does Lead Have A High Specific Heat Capacity.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources Why Does Lead Have A High Specific Heat Capacity Water has a particularly high specific heat compared to many other substances. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat, or specific heat capacity, is a property related to internal. Specific heat of lead is 0.13 j/g k. 71 rows the specific heat is the amount of heat energy per unit mass. Why Does Lead Have A High Specific Heat Capacity.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Why Does Lead Have A High Specific Heat Capacity Specific heat, or specific heat capacity, is a property related to internal. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Deduce which substance will have greatest. 71 rows the specific heat is the amount of heat energy per unit mass required. Why Does Lead Have A High Specific Heat Capacity.
From www.linstitute.net
AQA A Level Physics复习笔记6.4.3 Specific Heat Capacity翰林国际教育 Why Does Lead Have A High Specific Heat Capacity Deduce which substance will have greatest. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat of lead is 0.13 j/g k. Specific heat, or specific heat capacity, is a property related to internal. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by. Why Does Lead Have A High Specific Heat Capacity.
From rosaqocuevas.blogspot.com
High Specific Heat Capacity RosaqoCuevas Why Does Lead Have A High Specific Heat Capacity This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Water has a particularly high specific heat compared to many. Why Does Lead Have A High Specific Heat Capacity.
From www.numerade.com
SOLVED Table 9.2 Specific Heat Capacities of Some Common Substances Specific Heat Capacity; Cs Why Does Lead Have A High Specific Heat Capacity Specific heat of lead is 0.13 j/g k. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Define heat capacity and specific heat capacity and differentiate between the two terms ; Its specific heat capacity is 4.184 j/g°c, which means it takes. Why Does Lead Have A High Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID3439007 Why Does Lead Have A High Specific Heat Capacity Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat, or specific heat capacity, is a property related to internal. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Water has a particularly high specific heat compared to many other. Why Does Lead Have A High Specific Heat Capacity.
From www.youtube.com
Specific heat capacity YouTube Why Does Lead Have A High Specific Heat Capacity Water has a particularly high specific heat compared to many other substances. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one. Why Does Lead Have A High Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID3951196 Why Does Lead Have A High Specific Heat Capacity This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Specific heat of lead is 0.13 j/g k. Deduce which. Why Does Lead Have A High Specific Heat Capacity.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity Why Does Lead Have A High Specific Heat Capacity 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Specific heat of lead is 0.13 j/g k. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Deduce which. Why Does Lead Have A High Specific Heat Capacity.
From mechanicvisualizes.z14.web.core.windows.net
If Something Has A High Specific Heat Why Does Lead Have A High Specific Heat Capacity Specific heat, or specific heat capacity, is a property related to internal. Deduce which substance will have greatest. Define heat capacity and specific heat capacity and differentiate between the two terms ; Specific heat of lead is 0.13 j/g k. Water has a particularly high specific heat compared to many other substances. Its specific heat capacity is 4.184 j/g°c, which. Why Does Lead Have A High Specific Heat Capacity.
From www.studypool.com
SOLUTION Specific heat capacity of different materials Studypool Why Does Lead Have A High Specific Heat Capacity 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Define heat capacity and specific heat capacity and differentiate between the two terms ; This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and. Why Does Lead Have A High Specific Heat Capacity.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube Why Does Lead Have A High Specific Heat Capacity Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat, or specific heat capacity, is a property related to internal. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Water has a particularly high specific heat compared to many other. Why Does Lead Have A High Specific Heat Capacity.
From www.markedbyteachers.com
Specific Heat Capacity International Baccalaureate Physics Marked by Why Does Lead Have A High Specific Heat Capacity Deduce which substance will have greatest. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Its specific heat capacity. Why Does Lead Have A High Specific Heat Capacity.
From www.slideserve.com
PPT Chapter 3 Matter and Energy PowerPoint Presentation, free download ID6999373 Why Does Lead Have A High Specific Heat Capacity This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Water has a particularly high specific heat compared to many. Why Does Lead Have A High Specific Heat Capacity.
From www.youtube.com
Specific Heat and Heat Capacity YouTube Why Does Lead Have A High Specific Heat Capacity Water has a particularly high specific heat compared to many other substances. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Specific heat, or specific heat capacity, is a property related to internal. Define heat capacity and specific heat capacity and differentiate. Why Does Lead Have A High Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID6613953 Why Does Lead Have A High Specific Heat Capacity Deduce which substance will have greatest. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Specific heat of lead is 0.13 j/g k. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and. Why Does Lead Have A High Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID3439007 Why Does Lead Have A High Specific Heat Capacity Specific heat of lead is 0.13 j/g k. Define heat capacity and specific heat capacity and differentiate between the two terms ; Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Water has a particularly high specific heat compared to many other substances. This explanation explains why the very simple nobles gases with almost none. Why Does Lead Have A High Specific Heat Capacity.
From jahamattbrown.blogspot.com
Specific Heat Capacity Definition Matt Brown Why Does Lead Have A High Specific Heat Capacity Specific heat, or specific heat capacity, is a property related to internal. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Define heat capacity and specific heat capacity and differentiate between the two terms ; Specific heat of lead is 0.13 j/g. Why Does Lead Have A High Specific Heat Capacity.
From www.studypool.com
SOLUTION Physics notes heat capacity and specific heat capacity Studypool Why Does Lead Have A High Specific Heat Capacity This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Specific heat, or specific heat capacity, is a property related to internal. Water has a particularly high specific heat compared to many other substances. Its specific heat capacity is 4.184 j/g°c, which means. Why Does Lead Have A High Specific Heat Capacity.
From slideplayer.com
Specific Heat Capacity ppt download Why Does Lead Have A High Specific Heat Capacity Deduce which substance will have greatest. Specific heat, or specific heat capacity, is a property related to internal. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Define heat capacity and specific heat capacity and differentiate between the two terms ; 71. Why Does Lead Have A High Specific Heat Capacity.
From slideplayer.com
Specific Heat Capacity & Calorimetry ppt download Why Does Lead Have A High Specific Heat Capacity Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Specific heat, or specific heat capacity, is a property related to internal. This explanation explains why the very simple nobles gases with almost. Why Does Lead Have A High Specific Heat Capacity.
From slideplayer.com
specific heat capacity ppt download Why Does Lead Have A High Specific Heat Capacity Specific heat of lead is 0.13 j/g k. 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Deduce which substance will have greatest. Specific heat, or specific heat capacity, is a property related to internal. Its specific heat capacity is 4.184 j/g°c, which means it takes. Why Does Lead Have A High Specific Heat Capacity.
From thechemistrynotes.com
Heat Capacity and Specific Heat Why Does Lead Have A High Specific Heat Capacity Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Specific heat, or specific heat capacity, is a property related to internal. Define heat capacity and specific heat capacity and differentiate between the two terms ; Water has a particularly high specific heat compared to many other substances. 71 rows the specific heat is the amount. Why Does Lead Have A High Specific Heat Capacity.
From studymind.co.uk
Heat Capacity Study Mind Why Does Lead Have A High Specific Heat Capacity 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Deduce which substance will have greatest. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate. Why Does Lead Have A High Specific Heat Capacity.
From slidetodoc.com
specific heat Capacity EQ What is specific heat Why Does Lead Have A High Specific Heat Capacity Define heat capacity and specific heat capacity and differentiate between the two terms ; Deduce which substance will have greatest. Specific heat of lead is 0.13 j/g k. Specific heat, or specific heat capacity, is a property related to internal. Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. 71 rows the specific heat is. Why Does Lead Have A High Specific Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID5118755 Why Does Lead Have A High Specific Heat Capacity 71 rows the specific heat is the amount of heat energy per unit mass required to raise the temperature by one degree celsius. Specific heat of lead is 0.13 j/g k. Specific heat, or specific heat capacity, is a property related to internal. This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate. Why Does Lead Have A High Specific Heat Capacity.
From slideplayer.com
Specific Heat Capacity (比熱容量) ppt download Why Does Lead Have A High Specific Heat Capacity This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar) heat capacity and why carbon dioxide or. Deduce which substance will have greatest. Specific heat, or specific heat capacity, is a property related to internal. Water has a particularly high specific heat compared to many other substances. 71 rows the. Why Does Lead Have A High Specific Heat Capacity.
From studylib.net
1.3 Specific Heat Capacity Why Does Lead Have A High Specific Heat Capacity Its specific heat capacity is 4.184 j/g°c, which means it takes 4.184 joules of. Water has a particularly high specific heat compared to many other substances. Define heat capacity and specific heat capacity and differentiate between the two terms ; This explanation explains why the very simple nobles gases with almost none degrees of freedom to vibrate have low (molar). Why Does Lead Have A High Specific Heat Capacity.