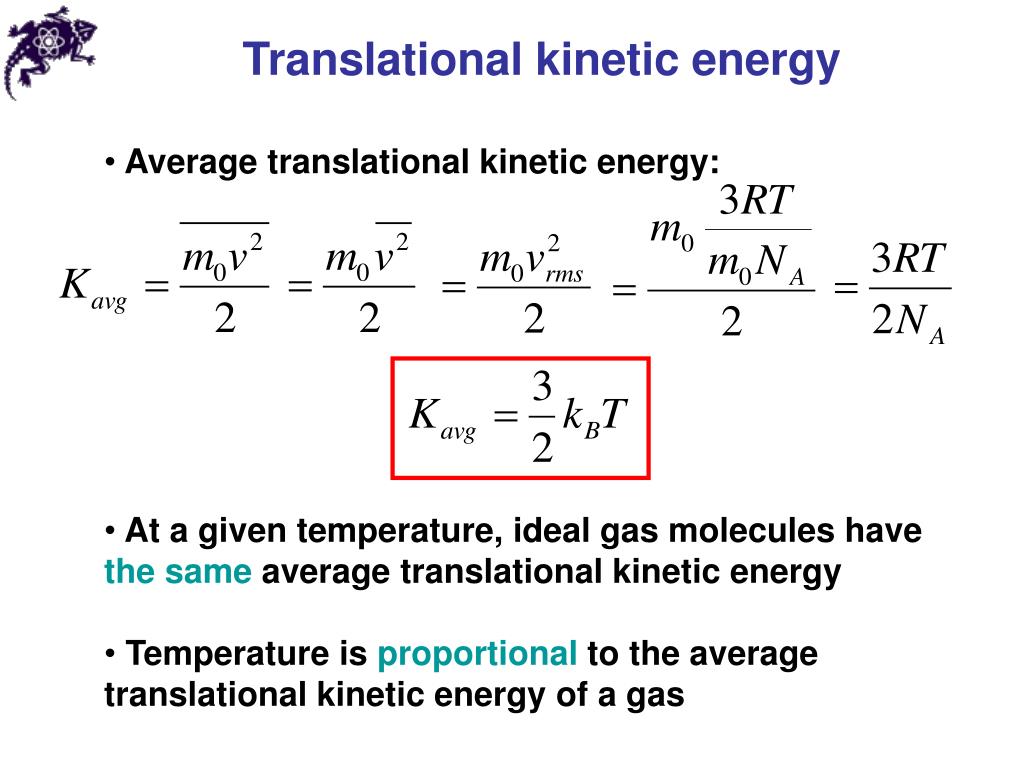
How Heat Is Kinetic Energy . Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Thermal energy, in turn, is the kinetic energy of. 2.1relative motion, distance, and displacement. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. The kinetic theory helps us understand where the energy goes when we heat something up. Temperature is the average kinetic. Only the kinetic energy of their disordered movement is. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy. If you put a pan full of cold water on a hot stove,. Yes, heat is kinetic energy of the atoms and molecules.
from www.slideserve.com
If you put a pan full of cold water on a hot stove,. 2.1relative motion, distance, and displacement. The kinetic theory helps us understand where the energy goes when we heat something up. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Only the kinetic energy of their disordered movement is. Thermal energy, in turn, is the kinetic energy of. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. Temperature is the average kinetic. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy.
PPT Chapter 10 Thermal Physics PowerPoint Presentation, free download
How Heat Is Kinetic Energy Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. 2.1relative motion, distance, and displacement. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Yes, heat is kinetic energy of the atoms and molecules. Only the kinetic energy of their disordered movement is. If you put a pan full of cold water on a hot stove,. The kinetic theory helps us understand where the energy goes when we heat something up. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. Thermal energy, in turn, is the kinetic energy of. Temperature is the average kinetic. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy.
From slideplayer.com
Temperature Section ppt download How Heat Is Kinetic Energy Only the kinetic energy of their disordered movement is. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. If you put a pan full of cold water on a hot stove,. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume. How Heat Is Kinetic Energy.
From www.vecteezy.com
Energy and Temperature science vector graphic 21790106 Vector How Heat Is Kinetic Energy Yes, heat is kinetic energy of the atoms and molecules. The kinetic theory helps us understand where the energy goes when we heat something up. If you put a pan full of cold water on a hot stove,. Only the kinetic energy of their disordered movement is. 2.1relative motion, distance, and displacement. The temperature of an object relates to both. How Heat Is Kinetic Energy.
From quizlet.com
Thermal, and Potential Energy Diagram Quizlet How Heat Is Kinetic Energy The temperature of an object relates to both the kinetic energy of its particles and the number of particles. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy. If you put a pan full of cold water on. How Heat Is Kinetic Energy.
From www.dreamstime.com
Thermal Energy Physics Definition, Example with Water and How Heat Is Kinetic Energy Yes, heat is kinetic energy of the atoms and molecules. 2.1relative motion, distance, and displacement. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy. How Heat Is Kinetic Energy.
From www.youtube.com
6.1 Temperature and energy (SL) YouTube How Heat Is Kinetic Energy Temperature is the average kinetic. If you put a pan full of cold water on a hot stove,. 2.1relative motion, distance, and displacement. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Thermal energy, in turn, is the kinetic energy of. The kinetic theory helps us understand where the. How Heat Is Kinetic Energy.
From sciencenotes.org
What Is Energy? Energy Examples How Heat Is Kinetic Energy If you put a pan full of cold water on a hot stove,. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy. Thermal energy, in turn, is the kinetic energy of. Temperature is the average kinetic. Heat is. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT What is the difference between heat and temperature? PowerPoint How Heat Is Kinetic Energy Heat is the thermal energy transfer between systems or bodies due to a temperature difference. If you put a pan full of cold water on a hot stove,. 2.1relative motion, distance, and displacement. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT SALADIN C. 26 PowerPoint Presentation, free download ID3267585 How Heat Is Kinetic Energy 2.1relative motion, distance, and displacement. Thermal energy, in turn, is the kinetic energy of. Yes, heat is kinetic energy of the atoms and molecules. If you put a pan full of cold water on a hot stove,. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The kinetic theory helps us understand where the. How Heat Is Kinetic Energy.
From www.studypool.com
SOLUTION Heat energy and thermodynamics mcqs Studypool How Heat Is Kinetic Energy 2.1relative motion, distance, and displacement. Temperature is the average kinetic. If you put a pan full of cold water on a hot stove,. The kinetic theory helps us understand where the energy goes when we heat something up. Thermal energy, in turn, is the kinetic energy of. Heat is the thermal energy transfer between systems or bodies due to a. How Heat Is Kinetic Energy.
From www.youtube.com
Energy, Temperature and Particle Speeds YouTube How Heat Is Kinetic Energy As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy. 2.1relative motion, distance, and displacement. Only the kinetic energy of their disordered movement is. The kinetic theory helps us understand where the energy goes when we heat something up.. How Heat Is Kinetic Energy.
From www.pinterest.co.uk
Types and forms of energy. potential, mechanical, chemical How Heat Is Kinetic Energy The kinetic theory helps us understand where the energy goes when we heat something up. Only the kinetic energy of their disordered movement is. 2.1relative motion, distance, and displacement. If you put a pan full of cold water on a hot stove,. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The temperature of. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Temperature v. Heat PowerPoint Presentation ID1701293 How Heat Is Kinetic Energy The temperature of an object relates to both the kinetic energy of its particles and the number of particles. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Potential and energy PowerPoint Presentation, free How Heat Is Kinetic Energy Temperature is the average kinetic. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The kinetic theory helps us understand where the energy goes when we heat something up. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic. How Heat Is Kinetic Energy.
From webapi.bu.edu
💌 Thermal energy energy. What Are the Differences Between How Heat Is Kinetic Energy The kinetic theory helps us understand where the energy goes when we heat something up. Thermal energy, in turn, is the kinetic energy of. Only the kinetic energy of their disordered movement is. 2.1relative motion, distance, and displacement. Temperature is the average kinetic. If you put a pan full of cold water on a hot stove,. As the object comes. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Physical Science Chapter 6 PowerPoint Presentation, free download How Heat Is Kinetic Energy Temperature is the average kinetic. If you put a pan full of cold water on a hot stove,. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. Thermal energy, in turn, is the kinetic energy of. Heat is the thermal energy transfer between systems or bodies due to a temperature. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Heat & Thermodynamics PowerPoint Presentation, free download ID How Heat Is Kinetic Energy Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Thermal energy, in turn, is the kinetic energy of. Only the kinetic energy of their disordered movement is. The kinetic theory helps us understand where. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Chapter 10 Thermal Physics PowerPoint Presentation, free download How Heat Is Kinetic Energy Thermal energy, in turn, is the kinetic energy of. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. If you put a pan full of cold water on a hot stove,. Temperature is the average kinetic. The temperature of an object relates to both the kinetic energy of its. How Heat Is Kinetic Energy.
From slideplayer.com
Heat A Form of Energy. ppt download How Heat Is Kinetic Energy Only the kinetic energy of their disordered movement is. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. The kinetic theory helps us understand where the energy goes when we heat something up. As the object comes to rest, its kinetic energy appears as heat (in both the object. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Unit 6 PowerPoint Presentation, free download ID1516058 How Heat Is Kinetic Energy Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Yes, heat is kinetic energy of the atoms and molecules. Thermal energy, in turn, is the kinetic energy of. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Only the kinetic energy of their. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT HEAT and HEAT TRANSFER PowerPoint Presentation, free download How Heat Is Kinetic Energy The kinetic theory helps us understand where the energy goes when we heat something up. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy. Thermal energy, in turn, is the kinetic energy of. Heat is the thermal energy. How Heat Is Kinetic Energy.
From www.pinterest.cl
The 2 types and 9 forms of Energy and Potential Learn How Heat Is Kinetic Energy 2.1relative motion, distance, and displacement. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. The kinetic theory helps us understand where the energy goes when we heat something up. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. As the object comes to rest,. How Heat Is Kinetic Energy.
From thepowerfacts.com
Is Heat Energy Potential Or (Answer is Here) The Power Facts How Heat Is Kinetic Energy Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. As the object comes to rest,. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Thermal Energy A. Temperature & Heat 1. Temperature is related to How Heat Is Kinetic Energy Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. 2.1relative motion, distance, and displacement. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy. Thermal energy, in turn,. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Thermodynamics the study of thermal energy PowerPoint How Heat Is Kinetic Energy Temperature is the average kinetic. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy. Thermal energy, in turn, is. How Heat Is Kinetic Energy.
From engineerfix.com
What Is Energy? Definition, Examples, Equation, and FAQs How Heat Is Kinetic Energy As the object comes to rest, its kinetic energy appears as heat (in both the object itself and in the table top) as the kinetic energy becomes randomized as thermal energy. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Yes, heat is kinetic energy of the atoms and molecules. Only the kinetic energy. How Heat Is Kinetic Energy.
From slideplayer.com
Chapter 3 Water and Life ppt download How Heat Is Kinetic Energy Temperature is the average kinetic. Yes, heat is kinetic energy of the atoms and molecules. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Only the kinetic energy of their. How Heat Is Kinetic Energy.
From sciencing.com
Thermal Energy Definition, Equation, Types (w/ Diagram & Examples How Heat Is Kinetic Energy 2.1relative motion, distance, and displacement. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. The kinetic theory helps us understand where the energy goes when we heat something up. Only the kinetic energy of. How Heat Is Kinetic Energy.
From engineerfix.com
What Is Energy? Definition, Examples, Equation, and FAQs How Heat Is Kinetic Energy Thermal energy, in turn, is the kinetic energy of. If you put a pan full of cold water on a hot stove,. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Only the kinetic. How Heat Is Kinetic Energy.
From www.sciencefacts.net
Thermal (Heat) Energy Definition, Examples, Equations, and Units How Heat Is Kinetic Energy Only the kinetic energy of their disordered movement is. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. If you put a pan full of cold water on a hot stove,. The kinetic theory helps us understand where the energy goes when we heat something up. Yes, heat is kinetic. How Heat Is Kinetic Energy.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii How Heat Is Kinetic Energy Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. The kinetic theory helps us understand where the energy goes when we heat something up. Thermal energy, in turn, is the kinetic energy of. Only the kinetic energy of their disordered movement is. Heat is the thermal energy transfer between. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT HEAT and HEAT TRANSFER PowerPoint Presentation, free download How Heat Is Kinetic Energy The kinetic theory helps us understand where the energy goes when we heat something up. Yes, heat is kinetic energy of the atoms and molecules. Temperature is the average kinetic. Only the kinetic energy of their disordered movement is. The temperature of an object relates to both the kinetic energy of its particles and the number of particles. If you. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID3521096 How Heat Is Kinetic Energy Only the kinetic energy of their disordered movement is. Thermal energy, in turn, is the kinetic energy of. The kinetic theory helps us understand where the energy goes when we heat something up. Learn about kinetic theory, which includes using the celsius and kelvin scales, the relationship between pressure, temperature and volume in. The temperature of an object relates to. How Heat Is Kinetic Energy.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential How Heat Is Kinetic Energy The kinetic theory helps us understand where the energy goes when we heat something up. Yes, heat is kinetic energy of the atoms and molecules. Temperature is the average kinetic. If you put a pan full of cold water on a hot stove,. Thermal energy, in turn, is the kinetic energy of. As the object comes to rest, its kinetic. How Heat Is Kinetic Energy.
From www.slideserve.com
PPT Energy! PowerPoint Presentation ID2954517 How Heat Is Kinetic Energy Thermal energy, in turn, is the kinetic energy of. Temperature is the average kinetic. The kinetic theory helps us understand where the energy goes when we heat something up. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. As the object comes to rest, its kinetic energy appears as heat (in both the object. How Heat Is Kinetic Energy.
From www.youtube.com
Chapter 10 21 Energy is Related to Temperature YouTube How Heat Is Kinetic Energy The temperature of an object relates to both the kinetic energy of its particles and the number of particles. Yes, heat is kinetic energy of the atoms and molecules. Heat is the thermal energy transfer between systems or bodies due to a temperature difference. Temperature is the average kinetic. Thermal energy, in turn, is the kinetic energy of. If you. How Heat Is Kinetic Energy.