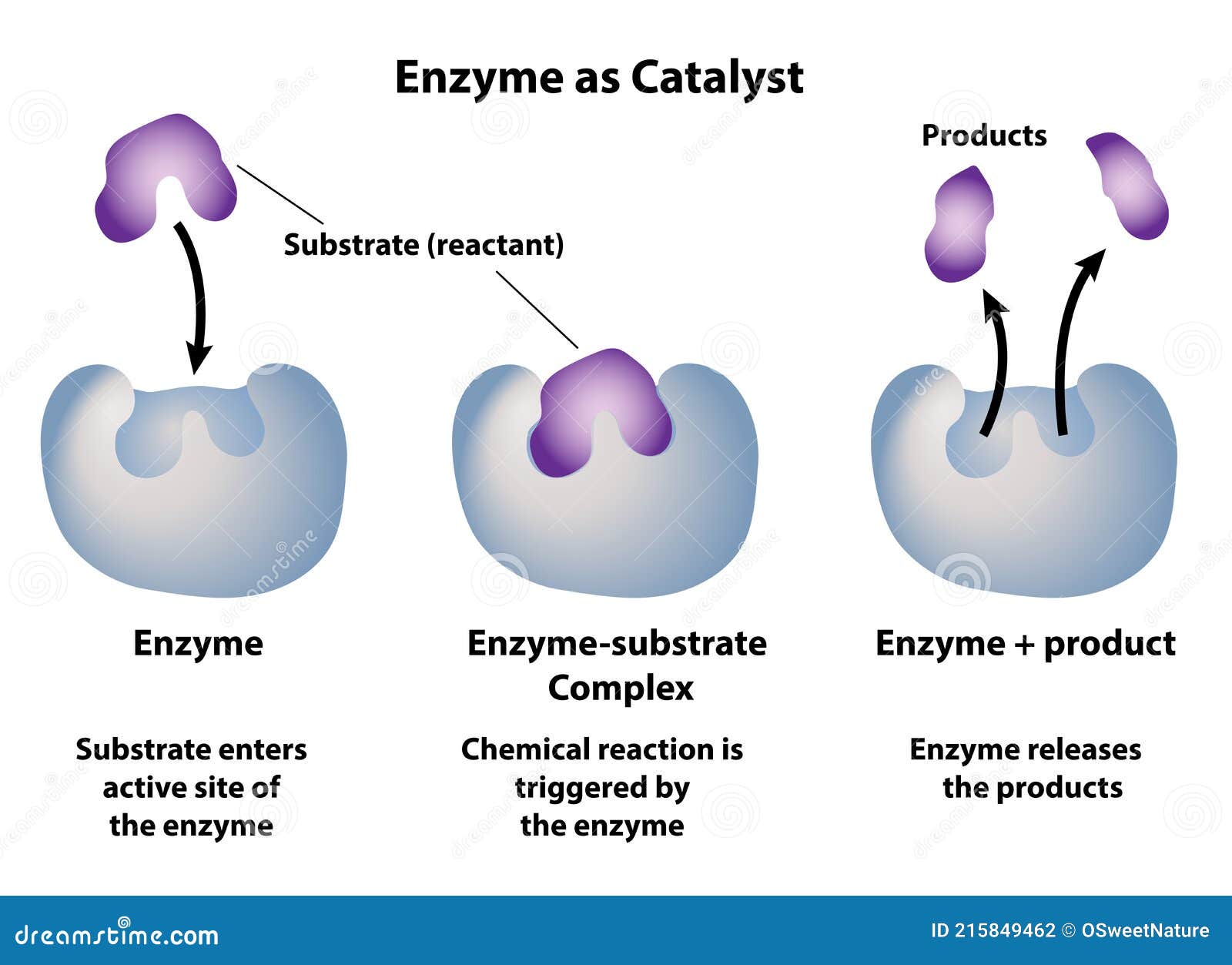
Why Isn't A Catalyst A Reactant . A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts allow a reaction to. The catalyst is not used up or chemically changed during the reaction. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. With a catalyst present, more collisions between reactants result in the. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst is some material that speeds up chemical reactions. Catalysts participate in a chemical reaction and increase its rate. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. They do not appear in the reaction’s net equation and are not consumed during the reaction. Factories rely on catalysts to make everything from plastic to drugs. Catalysts help process petroleum and coal into liquid fuels. A catalyst is a substance that speeds up a chemical reaction. Enzymes are catalysts that work inside the cells of.
from www.dreamstime.com
With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Factories rely on catalysts to make everything from plastic to drugs. It lowers the activation energy. A catalyst is some material that speeds up chemical reactions. The catalyst is not used up or chemically changed during the reaction. Catalysts allow a reaction to. With a catalyst present, more collisions between reactants result in the. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalysts participate in a chemical reaction and increase its rate.
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of
Why Isn't A Catalyst A Reactant Enzymes are catalysts that work inside the cells of. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. With a catalyst present, more collisions between reactants result in the. Catalysts participate in a chemical reaction and increase its rate. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Catalysts allow a reaction to. A catalyst is a substance that speeds up a chemical reaction. Factories rely on catalysts to make everything from plastic to drugs. The catalyst is not used up or chemically changed during the reaction. Catalysts help process petroleum and coal into liquid fuels. Enzymes are catalysts that work inside the cells of. A catalyst is some material that speeds up chemical reactions. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. It lowers the activation energy.
From www.slideserve.com
PPT Reaction Mechanism PowerPoint Presentation, free download ID Why Isn't A Catalyst A Reactant Catalysts help process petroleum and coal into liquid fuels. A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts allow a reaction to. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered. Why Isn't A Catalyst A Reactant.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Why Isn't A Catalyst A Reactant Catalysts allow a reaction to. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. Enzymes are catalysts that work inside the cells of. It lowers the activation energy. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. Why Isn't A Catalyst A Reactant.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Why Isn't A Catalyst A Reactant Catalysts allow a reaction to. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst reduces the activation energy needed for a reaction. Factories rely on catalysts to make everything from plastic to drugs. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant,. Why Isn't A Catalyst A Reactant.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Why Isn't A Catalyst A Reactant Catalysts allow a reaction to. A catalyst reduces the activation energy needed for a reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. With a catalyst present, more collisions between reactants result in the. A catalyst is some material that speeds up chemical reactions. Factories rely on catalysts to make everything from. Why Isn't A Catalyst A Reactant.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Why Isn't A Catalyst A Reactant A catalyst reduces the activation energy needed for a reaction. A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. Enzymes are catalysts that work inside the cells of. With a helping hand from a catalyst, molecules that might take years to interact. Why Isn't A Catalyst A Reactant.
From helecu.com
Factors Affecting Reaction Rates Chemistry Atoms First (2022) Why Isn't A Catalyst A Reactant Catalysts allow a reaction to. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. Enzymes are catalysts that work inside the cells of. Factories rely on catalysts to make everything from plastic to drugs. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance that speeds up. Why Isn't A Catalyst A Reactant.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Why Isn't A Catalyst A Reactant Enzymes are catalysts that work inside the cells of. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst reduces the activation energy needed for a reaction. Catalysts. Why Isn't A Catalyst A Reactant.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Why Isn't A Catalyst A Reactant Catalysts help process petroleum and coal into liquid fuels. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. With a catalyst present, more collisions between reactants result in the. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst binds to. Why Isn't A Catalyst A Reactant.
From www.slideserve.com
PPT Part IV Activation Energy Jespersen Chapter 14 Sec 5 Why Isn't A Catalyst A Reactant With a catalyst present, more collisions between reactants result in the. The catalyst is not used up or chemically changed during the reaction. Catalysts participate in a chemical reaction and increase its rate. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. Factories rely on catalysts. Why Isn't A Catalyst A Reactant.
From slideplayer.com
Rates of reactions A car rusting is a slow reaction it takes a long Why Isn't A Catalyst A Reactant Factories rely on catalysts to make everything from plastic to drugs. The catalyst is not used up or chemically changed during the reaction. Enzymes are catalysts that work inside the cells of. A catalyst reduces the activation energy needed for a reaction. Catalysts help process petroleum and coal into liquid fuels. They do not appear in the reaction’s net equation. Why Isn't A Catalyst A Reactant.
From www.numerade.com
SOLVED E 1 Reaction Progress The diagram above shows the reaction Why Isn't A Catalyst A Reactant A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. It lowers the activation energy. A catalyst is some material that speeds up chemical reactions. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst reduces the activation energy needed for. Why Isn't A Catalyst A Reactant.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Why Isn't A Catalyst A Reactant A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. The catalyst is not used up or chemically changed during the reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst binds to a reactant and. Why Isn't A Catalyst A Reactant.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Why Isn't A Catalyst A Reactant The catalyst is not used up or chemically changed during the reaction. A catalyst reduces the activation energy needed for a reaction. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. Catalysts help process petroleum and coal into liquid fuels. A catalyst is some material that speeds up chemical reactions. A catalyst. Why Isn't A Catalyst A Reactant.
From ppt-online.org
Rates of reaction презентация онлайн Why Isn't A Catalyst A Reactant A catalyst reduces the activation energy needed for a reaction. Catalysts help process petroleum and coal into liquid fuels. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. The catalyst is not used up or chemically changed during the reaction. With a helping hand from a catalyst, molecules that. Why Isn't A Catalyst A Reactant.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Why Isn't A Catalyst A Reactant A catalyst is a substance that speeds up a chemical reaction. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up. Why Isn't A Catalyst A Reactant.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Why Isn't A Catalyst A Reactant Factories rely on catalysts to make everything from plastic to drugs. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. It lowers the activation energy. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. With. Why Isn't A Catalyst A Reactant.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Why Isn't A Catalyst A Reactant A catalyst reduces the activation energy needed for a reaction. Catalysts participate in a chemical reaction and increase its rate. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. If a catalyst is consumed in the reaction without getting produced again, the catalyst is. Why Isn't A Catalyst A Reactant.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Why Isn't A Catalyst A Reactant A catalyst is some material that speeds up chemical reactions. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. Enzymes are catalysts that work inside the cells of. A catalyst is a substance that speeds up a chemical reaction without being consumed in. Why Isn't A Catalyst A Reactant.
From www.ck12.org
Reaction Mechanisms Example 3 ( Video ) Chemistry CK12 Foundation Why Isn't A Catalyst A Reactant If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. They do not appear in the reaction’s net equation and are not consumed during the. Why Isn't A Catalyst A Reactant.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Why Isn't A Catalyst A Reactant Factories rely on catalysts to make everything from plastic to drugs. A catalyst is a substance that speeds up a chemical reaction. A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. The catalyst is not used up or chemically changed during the reaction. They do not appear in the reaction’s net equation. Why Isn't A Catalyst A Reactant.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Why Isn't A Catalyst A Reactant Catalysts help process petroleum and coal into liquid fuels. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Factories rely on catalysts to make everything from plastic to drugs. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the.. Why Isn't A Catalyst A Reactant.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent Why Isn't A Catalyst A Reactant Enzymes are catalysts that work inside the cells of. A catalyst reduces the activation energy needed for a reaction. A catalyst is some material that speeds up chemical reactions. A catalyst is a substance that speeds up a chemical reaction. Factories rely on catalysts to make everything from plastic to drugs. If a catalyst is consumed in the reaction without. Why Isn't A Catalyst A Reactant.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Why Isn't A Catalyst A Reactant With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Factories rely on catalysts to make everything from plastic to drugs. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is a substance that speeds up a chemical reaction. If a. Why Isn't A Catalyst A Reactant.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube Why Isn't A Catalyst A Reactant With a catalyst present, more collisions between reactants result in the. A catalyst is some material that speeds up chemical reactions. The catalyst is not used up or chemically changed during the reaction. Catalysts help process petroleum and coal into liquid fuels. It lowers the activation energy. If a catalyst is consumed in the reaction without getting produced again, the. Why Isn't A Catalyst A Reactant.
From slideplayer.com
Enzyme Activity The Digestive System. ppt video online download Why Isn't A Catalyst A Reactant Catalysts participate in a chemical reaction and increase its rate. It lowers the activation energy. With a catalyst present, more collisions between reactants result in the. A catalyst is a substance that speeds up a chemical reaction. Factories rely on catalysts to make everything from plastic to drugs. With a helping hand from a catalyst, molecules that might take years. Why Isn't A Catalyst A Reactant.
From chem.libretexts.org
Catalytic Hydrogenation of Alkenes Chemistry LibreTexts Why Isn't A Catalyst A Reactant With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts help process petroleum and coal into liquid fuels. A catalyst reduces the activation energy needed for a reaction. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used. Why Isn't A Catalyst A Reactant.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Why Isn't A Catalyst A Reactant A catalyst is some material that speeds up chemical reactions. Factories rely on catalysts to make everything from plastic to drugs. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without. Why Isn't A Catalyst A Reactant.
From essentialchemicalindustry.com
Catalysis in industry Why Isn't A Catalyst A Reactant A catalyst reduces the activation energy needed for a reaction. Factories rely on catalysts to make everything from plastic to drugs. Catalysts allow a reaction to. A catalyst is a substance that speeds up a chemical reaction. Catalysts help process petroleum and coal into liquid fuels. Enzymes are catalysts that work inside the cells of. It lowers the activation energy.. Why Isn't A Catalyst A Reactant.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 Why Isn't A Catalyst A Reactant A catalyst is a substance that speeds up a chemical reaction without being consumed in the process. Catalysts participate in a chemical reaction and increase its rate. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. If a catalyst is consumed in the reaction without getting produced again, the. Why Isn't A Catalyst A Reactant.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Why Isn't A Catalyst A Reactant A catalyst reduces the activation energy needed for a reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Enzymes are catalysts that work inside the cells of. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction.. Why Isn't A Catalyst A Reactant.
From sciencenotes.org
What Is a Reactant in Chemistry? Definition and Examples Why Isn't A Catalyst A Reactant Catalysts participate in a chemical reaction and increase its rate. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Factories rely on catalysts to make everything from plastic to drugs. It lowers the activation energy. A catalyst binds to a reactant and it increases the number of collision between. Why Isn't A Catalyst A Reactant.
From www.savemyexams.co.uk
Catalysts (1.7.6) AQA A Level Chemistry Revision Notes 2017 Save My Why Isn't A Catalyst A Reactant It lowers the activation energy. Factories rely on catalysts to make everything from plastic to drugs. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst is some material that speeds up chemical reactions. Enzymes are catalysts that work inside the cells of. Catalysts participate in a chemical reaction and increase its. Why Isn't A Catalyst A Reactant.
From 2012books.lardbucket.org
Catalysis Why Isn't A Catalyst A Reactant A catalyst reduces the activation energy needed for a reaction. A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed during the reaction. With a catalyst present, more collisions between reactants result in the. Enzymes are catalysts that work inside the cells of. A catalyst is some material that speeds. Why Isn't A Catalyst A Reactant.
From www.chemicalslearning.com
Types of Reactants in Chemistry Why Isn't A Catalyst A Reactant They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts help process petroleum and coal into liquid fuels. A catalyst reduces the activation energy needed for a reaction. If a catalyst is consumed in the reaction without getting produced again, the catalyst is considered a reactant, changing the. A catalyst binds to a. Why Isn't A Catalyst A Reactant.
From slideplayer.com
Collision Theory. ppt download Why Isn't A Catalyst A Reactant Catalysts allow a reaction to. A catalyst is a substance that speeds up a chemical reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more favorable thermodynamically. A catalyst is some material. Why Isn't A Catalyst A Reactant.