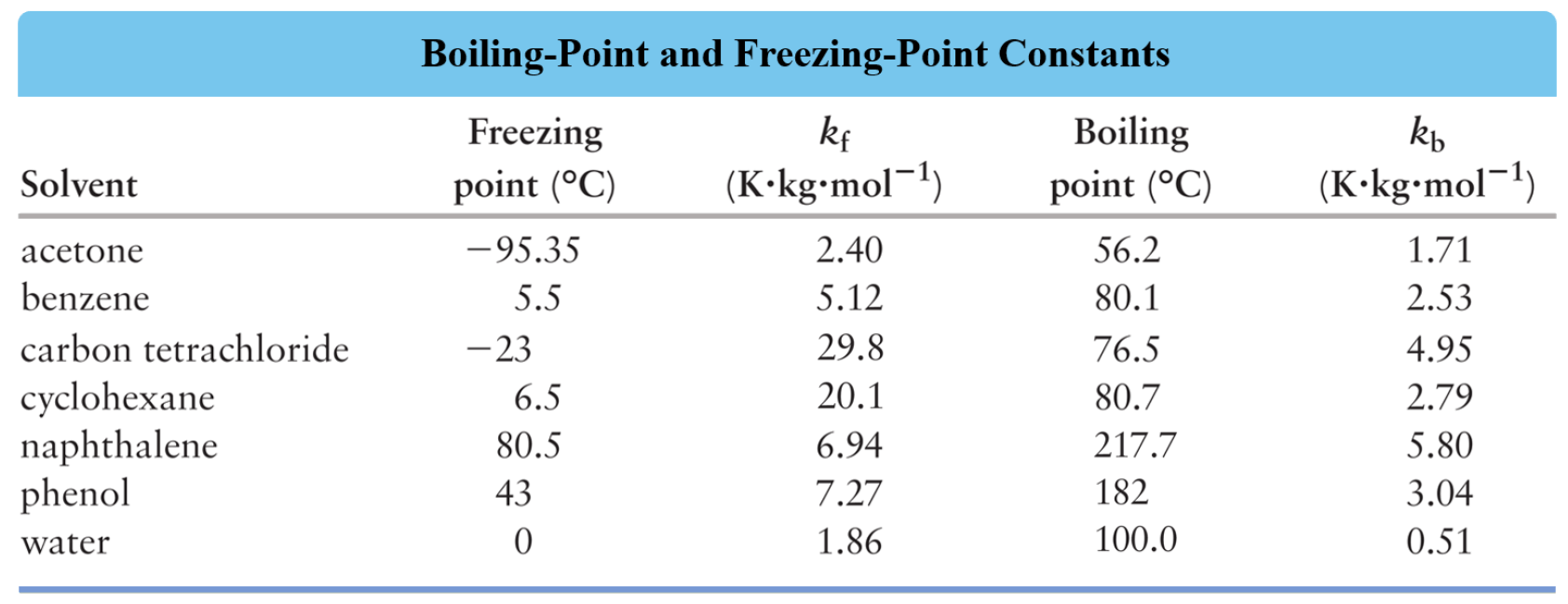
Boiling Point Of Pure Water Solutions . \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. A solution typically has a. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. For example, the boiling point of pure water at \(1.0 \: Note that the normal boiling.
from general.chemistrysteps.com
\text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Note that the normal boiling. The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. For example, the boiling point of pure water at \(1.0 \: A solution typically has a. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed.
Boiling Point Elevation Chemistry Steps
Boiling Point Of Pure Water Solutions For example, the boiling point of pure water at \(1.0 \: Note that the normal boiling. A solution typically has a. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. For example, the boiling point of pure water at \(1.0 \: The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling.
From www.toppr.com
(a) The boiling point of pure water is 373K. Calculate the boiling Boiling Point Of Pure Water Solutions Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. A solution typically has a. Note that the normal boiling. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. For example, the boiling point of pure water at. Boiling Point Of Pure Water Solutions.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID6009487 Boiling Point Of Pure Water Solutions \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. The boiling. Boiling Point Of Pure Water Solutions.
From uchart.web.app
Water Boiling Point Pressure Chart Boiling Point Of Pure Water Solutions A solution typically has a. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. The normal boiling point (also called the atmospheric boiling. Boiling Point Of Pure Water Solutions.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Boiling Point Of Pure Water Solutions The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. A solution typically has a. Note that the normal boiling. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding. Boiling Point Of Pure Water Solutions.
From preparatorychemistry.com
pH and Equilibrium Boiling Point Of Pure Water Solutions Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. For example, the boiling point of pure water at \(1.0 \: Note that the normal boiling. The boiling point. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved Determine the boiling point of an aqueous solution Boiling Point Of Pure Water Solutions For example, the boiling point of pure water at \(1.0 \: Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. Note that the normal boiling. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). A solution typically has a. The normal boiling point (also called the atmospheric boiling. Boiling Point Of Pure Water Solutions.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Of Pure Water Solutions For example, the boiling point of pure water at \(1.0 \: Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved Arrange the boiling points of the aqueous solutions, Boiling Point Of Pure Water Solutions Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. Note that the normal boiling. The boiling point of pure water is 100°c, but that boiling point can be. Boiling Point Of Pure Water Solutions.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Boiling Point Of Pure Water Solutions Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. For example, the boiling point of pure water at \(1.0 \: \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point. Boiling Point Of Pure Water Solutions.
From www.solutionspile.com
[Solved] Arrange the boiling points of the aqueous soluti Boiling Point Of Pure Water Solutions \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. A solution typically has a. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. The boiling point. Boiling Point Of Pure Water Solutions.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Boiling Point Of Pure Water Solutions Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. For example, the boiling point of pure water at \(1.0 \: The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. A solution typically has a. \text{atm}\) is \(100^\text{o}. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved The table below shows the boiling points of pure Boiling Point Of Pure Water Solutions Note that the normal boiling. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. A solution typically. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved 1. Determine the boiling point of pure water given Boiling Point Of Pure Water Solutions The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. For example, the boiling point of pure water at \(1.0 \: \text{atm}\) is \(100^\text{o} \text{c}\) while. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved Rank The Boiling Points Of The Aqueous Solutions B... Boiling Point Of Pure Water Solutions The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point). Boiling Point Of Pure Water Solutions.
From slideplayer.com
SOLUTIONS ppt download Boiling Point Of Pure Water Solutions \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Because the presence. Boiling Point Of Pure Water Solutions.
From psiberg.com
Colligative Properties of Solutions PSIBERG Boiling Point Of Pure Water Solutions The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. A solution typically has a. Note that the normal boiling. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. For example, the boiling point of pure water at. Boiling Point Of Pure Water Solutions.
From brainly.com
Arrange the boiling points of the aqueous solutions, relative to pure Boiling Point Of Pure Water Solutions Note that the normal boiling. A solution typically has a. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. For example, the boiling point of pure water at \(1.0 \: The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a. Boiling Point Of Pure Water Solutions.
From www.slideserve.com
PPT Chapter 5 Solutions PowerPoint Presentation, free download ID Boiling Point Of Pure Water Solutions Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. Note that the normal boiling. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. A solution typically has. Boiling Point Of Pure Water Solutions.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Of Pure Water Solutions Note that the normal boiling. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\).. Boiling Point Of Pure Water Solutions.
From www.numerade.com
SOLVED A solution of a nonvolatile solute in water has a boiling point Boiling Point Of Pure Water Solutions The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. For example, the boiling point of pure water at \(1.0 \: The boiling point of pure water. Boiling Point Of Pure Water Solutions.
From www.numerade.com
SOLVED C. BoilingPoint Elevation Table 10.6 (data) Boiling point of Boiling Point Of Pure Water Solutions For example, the boiling point of pure water at \(1.0 \: The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). A solution typically has a. Note that the normal boiling. Because the presence of solute particles decreases. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved The boiling point of water is 100.00∘C at 1 Boiling Point Of Pure Water Solutions \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). A solution typically has a. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Note that the normal boiling. For example, the boiling point of pure water at \(1.0 \: The boiling point of pure water is. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved The boiling point of pure water is 100 °C. The Boiling Point Of Pure Water Solutions A solution typically has a. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. For example, the boiling point of pure water at \(1.0 \: The boiling point of pure water is 100°c, but that boiling point can be. Boiling Point Of Pure Water Solutions.
From www.numerade.com
Arrange the boiling points of the aqueous solutions, relative to pure Boiling Point Of Pure Water Solutions The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. Note that the normal boiling. The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. The boiling point elevation (\(δt_b\)) and freezing point. Boiling Point Of Pure Water Solutions.
From askfilo.com
The normal boiling point of pure water is 373.15 K. Calculate the boiling.. Boiling Point Of Pure Water Solutions The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. For example, the boiling point of pure water at \(1.0 \: The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. The normal boiling. Boiling Point Of Pure Water Solutions.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation ID5410728 Boiling Point Of Pure Water Solutions \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). A solution typically has a. For example, the boiling point of pure water at \(1.0 \: Note that the normal boiling. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The boiling point of pure water is 100°c, but. Boiling Point Of Pure Water Solutions.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock Boiling Point Of Pure Water Solutions A solution typically has a. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. Because the presence of solute particles decreases the vapor. Boiling Point Of Pure Water Solutions.
From www.numerade.com
SOLVED Arrange the boiling points of the aqueous solutions relative to Boiling Point Of Pure Water Solutions Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). Note that the normal boiling. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. The boiling point. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved Arrange the boiling points of the aqueous solutions, Boiling Point Of Pure Water Solutions Note that the normal boiling. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). For example, the boiling point of pure water at \(1.0 \: A solution typically has a. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. Because the presence of solute particles decreases. Boiling Point Of Pure Water Solutions.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download Boiling Point Of Pure Water Solutions Note that the normal boiling. Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. A solution typically has a. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. For example, the boiling point of pure water at \(1.0. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved Part A Boiling Point Elevation Note for water is kb Boiling Point Of Pure Water Solutions Because the presence of solute particles decreases the vapor pressure of the liquid solvent, a higher temperature is needed. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. Note that the normal boiling. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are. Boiling Point Of Pure Water Solutions.
From www.chegg.com
Solved suppose the boiling point of pure water at h h Boiling Point Of Pure Water Solutions The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. A solution typically has a. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). Note that the normal boiling. The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding. Boiling Point Of Pure Water Solutions.
From www.youtube.com
COMPARING BOILING POINTS OF PURE SUBSTANCE YouTube Boiling Point Of Pure Water Solutions The boiling point of pure water is 100°c, but that boiling point can be elevated by the adding of a solute such as a salt. Note that the normal boiling. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. The normal boiling point (also called the atmospheric boiling. Boiling Point Of Pure Water Solutions.
From pakmcqs.com
Boiling point of pure water is __________? PakMcqs Boiling Point Of Pure Water Solutions A solution typically has a. Note that the normal boiling. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling. For example, the boiling point of pure. Boiling Point Of Pure Water Solutions.
From mavink.com
Water Boiling Point Pressure Chart Boiling Point Of Pure Water Solutions The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). Note that the normal boiling. For example, the boiling point of pure water at \(1.0 \: A solution typically has a. The boiling point elevation (\(δt_b\)) and. Boiling Point Of Pure Water Solutions.