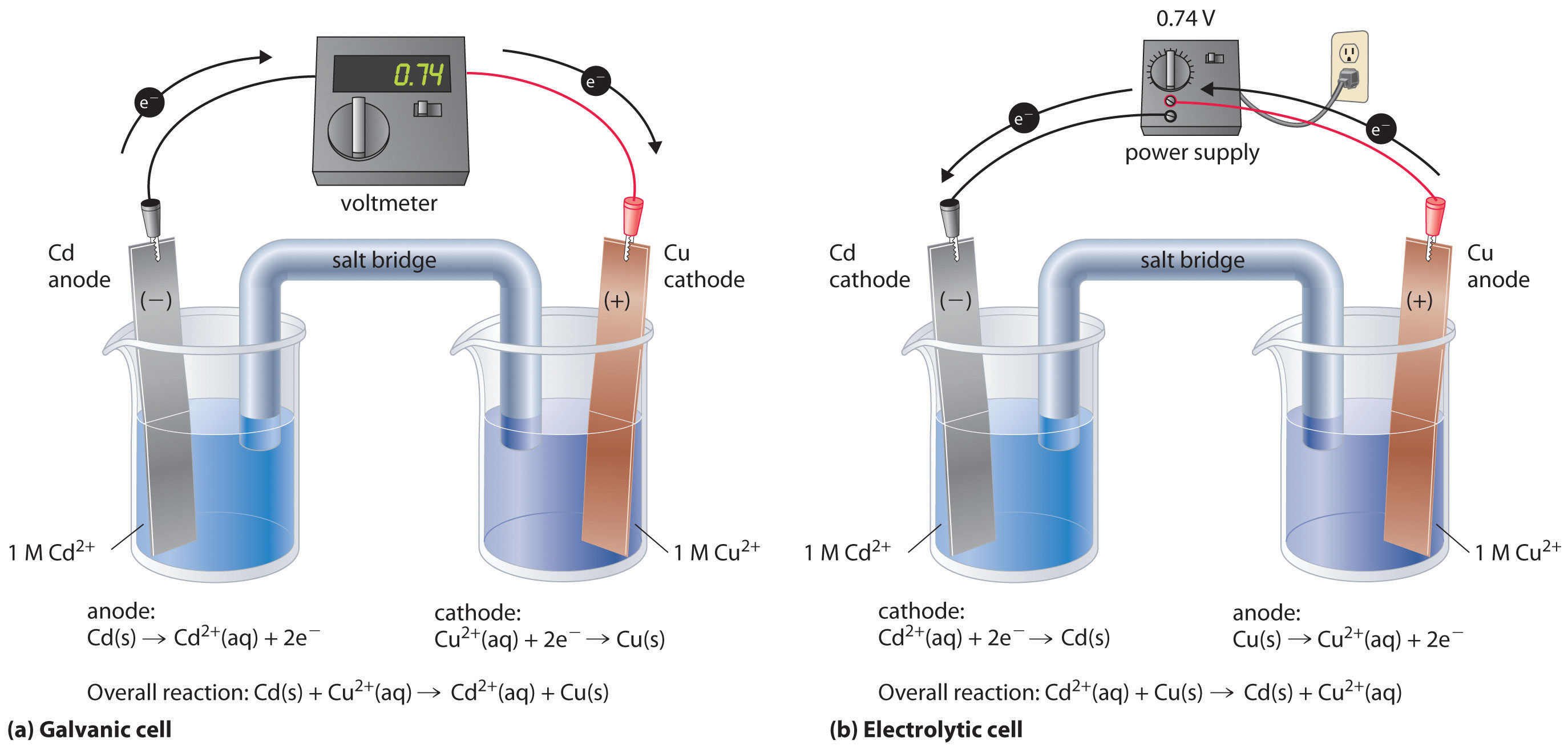
What Is Electrochemistry Cell . An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction.
from saylordotorg.github.io
An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after.
Electrochemistry
What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to.
From saylordotorg.github.io
Electrochemistry What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the. What Is Electrochemistry Cell.
From chemwiki.ucdavis.edu
Voltaic Cells Chemwiki What Is Electrochemistry Cell This kind of cell includes the galvanic, or voltaic, cell, named after. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is. What Is Electrochemistry Cell.
From www.youtube.com
Emf of daniel cell from nernst equation(Electrochemistry part 28 for What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is. What Is Electrochemistry Cell.
From mmerevise.co.uk
Electrochemical Cells MME What Is Electrochemistry Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An apparatus that is. What Is Electrochemistry Cell.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell What Is Electrochemistry Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell splits the oxidant and. What Is Electrochemistry Cell.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is. What Is Electrochemistry Cell.
From www.collegesearch.in
Electrochemical Cell Definitions, Examples, Electrochemistry What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current. What Is Electrochemistry Cell.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field What Is Electrochemistry Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is. What Is Electrochemistry Cell.
From www.youtube.com
Electrochemistry Classification Type Of Cell Electrochemical What Is Electrochemistry Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This kind. What Is Electrochemistry Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2281210 What Is Electrochemistry Cell This kind of cell includes the galvanic, or voltaic, cell, named after. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is. What Is Electrochemistry Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current. What Is Electrochemistry Cell.
From www.alamy.com
Basic components of an electrochemical cell Stock Photo Alamy What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells. What Is Electrochemistry Cell.
From www.youtube.com
electrochemical cell electrochemistry class 12 chemistry subject notes What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic,. What Is Electrochemistry Cell.
From studylib.net
Electrochemical cells What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic,. What Is Electrochemistry Cell.
From classfullbooklice.z13.web.core.windows.net
Working Of Electrochemical Cell What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons. What Is Electrochemistry Cell.
From wisc.pb.unizin.org
D39.4 Introduction to Voltaic Cells Chemistry 109 Fall 2021 What Is Electrochemistry Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is. What Is Electrochemistry Cell.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples What Is Electrochemistry Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An apparatus that is. What Is Electrochemistry Cell.
From www.vecteezy.com
Electrochemical cell or Galvanic cell, The Daniell cell 18989226 Vector What Is Electrochemistry Cell This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Voltaic cells produce electricity, while electrolytic cells use a power. An apparatus that is. What Is Electrochemistry Cell.
From greenenergymaterial.com
Basics Of Electrochemical Cells » Green Energy Material What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while. What Is Electrochemistry Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is. What Is Electrochemistry Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME What Is Electrochemistry Cell This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is. What Is Electrochemistry Cell.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and. What Is Electrochemistry Cell.
From www.thoughtco.com
Electrochemical Cell Definition What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is. What Is Electrochemistry Cell.
From www.alamy.com
Electrochemical cell or Galvanic cell, The Daniell cell with Voltmeter What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. What Is Electrochemistry Cell.
From www.slideshare.net
Electrochemistry electrochemical cells What Is Electrochemistry Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is. What Is Electrochemistry Cell.
From in.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram What Is Electrochemistry Cell An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind. What Is Electrochemistry Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell is a device that produces an electric current from energy released. What Is Electrochemistry Cell.
From enginelistmathew.z13.web.core.windows.net
Cathode In Electrochemical Cell What Is Electrochemistry Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. This kind of cell includes the galvanic,. What Is Electrochemistry Cell.
From www.pinterest.com
Electrochemical cell Electrochemistry, Chemistry classroom What Is Electrochemistry Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that. What Is Electrochemistry Cell.
From mavink.com
Diagram Of Electrochemical Cell What Is Electrochemistry Cell An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons. What Is Electrochemistry Cell.
From 2012books.lardbucket.org
Electrochemistry What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to. What Is Electrochemistry Cell.
From www.pearson.com
Electrochemical Cells Video Tutorial & Practice Channels for Pearson+ What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. An electrochemical cell is a device that produces an electric current. What Is Electrochemistry Cell.
From www.alamy.com
Educational Diagram of Chart showing Physics concept of Electrochemical What Is Electrochemistry Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or,. What Is Electrochemistry Cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and What Is Electrochemistry Cell Voltaic cells produce electricity, while electrolytic cells use a power. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is. What Is Electrochemistry Cell.
From saylordotorg.github.io
Electrochemistry What Is Electrochemistry Cell An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Voltaic cells produce electricity, while electrolytic cells use a power. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to. What Is Electrochemistry Cell.