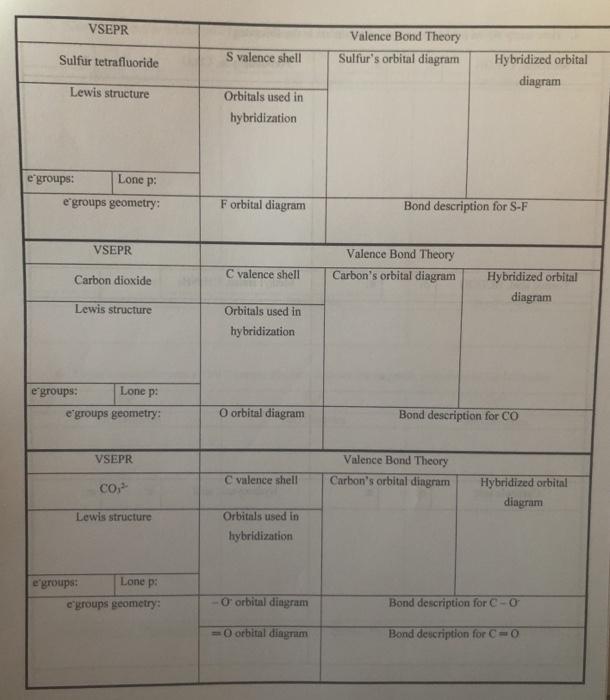
Bromine Pentafluoride Electron Pair Geometry . Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory.
from www.chegg.com
From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a.
Solved Bromine pentafluoride Electron Geometry Molecular
Bromine Pentafluoride Electron Pair Geometry Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a.
From exoiwchxo.blob.core.windows.net
Bromine Pentafluoride Electron Geometry at Tami Craig blog Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. From the lewis dot structure of brf5, it. Bromine Pentafluoride Electron Pair Geometry.
From www.clutchprep.com
Bromine pentafluoride, BrF5, is sometimes Clutch Prep Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). From the lewis dot structure of brf5, it is clear. Bromine Pentafluoride Electron Pair Geometry.
From exoiwchxo.blob.core.windows.net
Bromine Pentafluoride Electron Geometry at Tami Craig blog Bromine Pentafluoride Electron Pair Geometry The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. An explanation of the molecular. Bromine Pentafluoride Electron Pair Geometry.
From mungfali.com
Bromine Pentafluoride Lewis Structure Bromine Pentafluoride Electron Pair Geometry There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98.. Bromine Pentafluoride Electron Pair Geometry.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Stereochemical (In)activity Bromine Pentafluoride Electron Pair Geometry Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle. Bromine Pentafluoride Electron Pair Geometry.
From wellcometreeoflife.org
BrF3 Molecular Geometry (2021) Everything You Need to Know Bromine Pentafluoride Electron Pair Geometry An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle. Bromine Pentafluoride Electron Pair Geometry.
From www.wikiwand.com
Bromine pentafluoride Wikiwand Bromine Pentafluoride Electron Pair Geometry From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected. Bromine Pentafluoride Electron Pair Geometry.
From ajorpng.blogspot.com
Chlorine Pentafluoride Electron Pair Geometry Brf5 or bromine pentafluoride is a polar Bromine Pentafluoride Electron Pair Geometry An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair. Bromine Pentafluoride Electron Pair Geometry.
From www.numerade.com
SOLVED Draw the Lewis structure of bromine pentafluoride If your answer is a word, spelling Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. There are. Bromine Pentafluoride Electron Pair Geometry.
From gbu-taganskij.ru
Bromine Pentafluoride BrF5, The Formation Of [BrF6]−, 60 OFF Bromine Pentafluoride Electron Pair Geometry Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle. Bromine Pentafluoride Electron Pair Geometry.
From slideplayer.com
Molecular Geometry. ppt download Bromine Pentafluoride Electron Pair Geometry Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. Hence, the molecular geometry of brf 5 has only 90 degree. Bromine Pentafluoride Electron Pair Geometry.
From www.youtube.com
How to Draw the Lewis Dot Structure for BrF5 Bromine pentafluoride YouTube Bromine Pentafluoride Electron Pair Geometry From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. There are two molecular geometries that can come out of three. Bromine Pentafluoride Electron Pair Geometry.
From favpng.com
VSEPR Theory Tshaped Molecular Geometry Chemistry Lewis Pair Molecule, PNG, 628x600px, Vsepr Bromine Pentafluoride Electron Pair Geometry Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The difference between both the. Bromine Pentafluoride Electron Pair Geometry.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Stereochemical (In)activity Bromine Pentafluoride Electron Pair Geometry There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence. Bromine Pentafluoride Electron Pair Geometry.
From www.chegg.com
Solved Bromine pentafluoride Electron Geometry Molecular Bromine Pentafluoride Electron Pair Geometry Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. Hence, the molecular geometry of brf 5 has only 90 degree. Bromine Pentafluoride Electron Pair Geometry.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Stereochemical (In)activity Bromine Pentafluoride Electron Pair Geometry Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. Hence, the molecular geometry of brf 5 has only 90 degree. Bromine Pentafluoride Electron Pair Geometry.
From www.ck12.org
Molecular Geometry CK12 Foundation Bromine Pentafluoride Electron Pair Geometry Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. From the. Bromine Pentafluoride Electron Pair Geometry.
From exoiwchxo.blob.core.windows.net
Bromine Pentafluoride Electron Geometry at Tami Craig blog Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and. Bromine Pentafluoride Electron Pair Geometry.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Stereochemical (In)activity Bromine Pentafluoride Electron Pair Geometry The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle. Bromine Pentafluoride Electron Pair Geometry.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Stereochemical (In)activity Bromine Pentafluoride Electron Pair Geometry Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and. Bromine Pentafluoride Electron Pair Geometry.
From www.youtube.com
AP08 Lewis Dot structure bromine pentafluoride BrF5 YouTube Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. From the lewis dot structure of brf5, it. Bromine Pentafluoride Electron Pair Geometry.
From www.anyrgb.com
Bromine pentafluoride, phosphorus Tribromide, Nitrogen trichloride, phosphorus Pentafluoride Bromine Pentafluoride Electron Pair Geometry Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. An explanation of the molecular geometry for the brf5 (bromine pentafluoride). Bromine Pentafluoride Electron Pair Geometry.
From sciedutut.com
BrF5 Molecular Geometry Science Education and Tutorials Bromine Pentafluoride Electron Pair Geometry There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. From the lewis dot structure of brf5, it is clear that the electron. Bromine Pentafluoride Electron Pair Geometry.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Stereochemical (In)activity Bromine Pentafluoride Electron Pair Geometry Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. The molecular shape of brf. Bromine Pentafluoride Electron Pair Geometry.
From www.chegg.com
Solved Bromine pentafluoride Electron Geometry Molecular Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. From the lewis dot structure. Bromine Pentafluoride Electron Pair Geometry.
From www.numerade.com
SOLVED Answer the questions in the table below about the shape of the bromine pentafluoride Bromine Pentafluoride Electron Pair Geometry Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. From the lewis dot structure of brf5, it. Bromine Pentafluoride Electron Pair Geometry.
From www.vedantu.com
What is the geometry of the molecule of bromine pentafluoride?A.Square planarB.Trigonal Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. The molecular shape of brf 5 is square. Bromine Pentafluoride Electron Pair Geometry.
From www.slideserve.com
PPT VSEPR and Chemical Polarity PowerPoint Presentation, free download ID3318771 Bromine Pentafluoride Electron Pair Geometry From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule.. Bromine Pentafluoride Electron Pair Geometry.
From favpng.com
VSEPR Theory Chemistry Bromine Pentafluoride Electron Kugelwolkenmodell, PNG, 750x975px, Vsepr Bromine Pentafluoride Electron Pair Geometry The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. An explanation of the molecular geometry for the brf5 (bromine pentafluoride) including a. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. The difference between both the values is 1.02 which is. Bromine Pentafluoride Electron Pair Geometry.
From imgbin.com
Bromine Pentafluoride Lewis Structure Molecular Geometry Molecule PNG, Clipart, 2 D, Angle Bromine Pentafluoride Electron Pair Geometry Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. There are two molecular geometries that can come out of three electron domains, trigonal planar (no lone pairs) and bent with ≈ ≈ 120° bond angle (one lone pair). Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and. Bromine Pentafluoride Electron Pair Geometry.
From www.youtube.com
BrF5 Molecular Geometry & Bond Angles (Bromine Pentafluoride) YouTube Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values of bromine and fluorine are 2.96 and 3.98. An explanation of the molecular geometry for the. Bromine Pentafluoride Electron Pair Geometry.
From www.chegg.com
Solved Electron Geometry Molecular Geometry Bromine Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values. Bromine Pentafluoride Electron Pair Geometry.
From exoiwchxo.blob.core.windows.net
Bromine Pentafluoride Electron Geometry at Tami Craig blog Bromine Pentafluoride Electron Pair Geometry The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. Brf5 molecular. Bromine Pentafluoride Electron Pair Geometry.
From www.numerade.com
SOLVED Answer the questions in the table below about the shape of the bromine pentafluoride Bromine Pentafluoride Electron Pair Geometry Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. The molecular shape of brf 5 is square pyramidal, or ax 5 e using valence shell electron pair repulsion (vsepr) theory. From the lewis dot structure of brf5, it is clear that the electron geometry of the molecule is octahedral where the electronegativity values. Bromine Pentafluoride Electron Pair Geometry.
From quizlet.com
What is the hybridization of the central atom in the bromine Quizlet Bromine Pentafluoride Electron Pair Geometry Hence, the molecular geometry of brf 5 has only 90 degree bond angles in the molecule. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The difference between both the values is 1.02 which is greater than 0.4 so the brf5 molecule is a polar molecule. From the lewis dot structure. Bromine Pentafluoride Electron Pair Geometry.