Hcl Naoh Calorimetry . We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. Using a coffee cup calorimeter, the. The hcl and naoh then react until the solution temperature. Obtain four styrofoam cups and two plastic covers. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base.
from www.numerade.com
The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. The hcl and naoh then react until the solution temperature. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. Using a coffee cup calorimeter, the. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. Obtain four styrofoam cups and two plastic covers. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k.
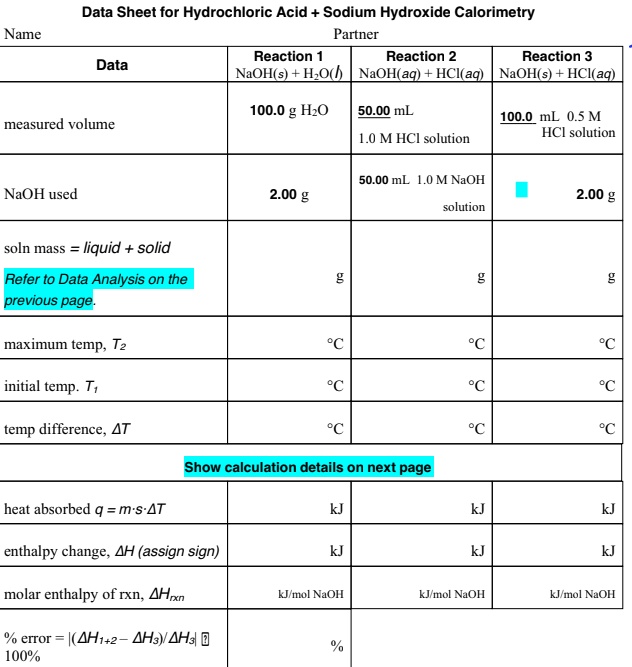
SOLVED Data Sheet for Hydrochloric Acid Sodium Hydroxide Calorimetry Partner Reaction Reaction
Hcl Naoh Calorimetry We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. The hcl and naoh then react until the solution temperature. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. Obtain four styrofoam cups and two plastic covers. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. Using a coffee cup calorimeter, the. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c.
From www.numerade.com
SOLVED QUESTION 7 Determining The Heat of Reaction Between NaOH and HCl Using An Insulated Hcl Naoh Calorimetry Obtain four styrofoam cups and two plastic covers. The hcl and naoh then react until the solution temperature. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. At the instant of mixing, you have 100.0. Hcl Naoh Calorimetry.
From www.slideserve.com
PPT Calorimetry Energetics PowerPoint Presentation, free download ID5975993 Hcl Naoh Calorimetry The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Obtain four styrofoam cups and two plastic covers. The hcl and naoh then react until. Hcl Naoh Calorimetry.
From www.numerade.com
SOLVED Data Sheet for Hydrochloric Acid Sodium Hydroxide Calorimetry Partner Reaction Reaction Hcl Naoh Calorimetry 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. Obtain four styrofoam cups and two plastic. Hcl Naoh Calorimetry.
From www.chegg.com
Solved Procedure 1. Calorimeter Has o. Procedure 2. NaOH/HCl Hcl Naoh Calorimetry The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. Using a coffee cup calorimeter, the. The hcl and naoh then react until the solution temperature. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45. Hcl Naoh Calorimetry.
From www.numerade.com
SOLVED 25.0 mL of 1.00 M HCl at 23.5°C react with 25.0 mL of 1.00 M NaOH in a styrofoam Hcl Naoh Calorimetry Obtain four styrofoam cups and two plastic covers. The hcl and naoh then react until the solution temperature. Using a coffee cup calorimeter, the. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. You will use either hcl or h 2 so 4 as your acid (you may choose.), and. Hcl Naoh Calorimetry.
From www.researchgate.net
(PDF) Specific Heat of Solution of HClNaOH Reaction by a Simple Handmade Calorimeter Hcl Naoh Calorimetry Using a coffee cup calorimeter, the. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. 2) place 50.0 ml of hcl and 50.0 ml of. Hcl Naoh Calorimetry.
From chart-studio.plotly.com
Heat of Neutralization for HCl with NaOH scatter chart made by Matthewwaller plotly Hcl Naoh Calorimetry You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. Using a coffee cup calorimeter, the. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c.. Hcl Naoh Calorimetry.
From www.numerade.com
SOLVED A quantity of 100 mL of a 0.500 M HCl was mixed with 100 mL of 0.500 M NaOH in a Hcl Naoh Calorimetry The hcl and naoh then react until the solution temperature. Using a coffee cup calorimeter, the. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Obtain four styrofoam cups and. Hcl Naoh Calorimetry.
From www.numerade.com
SOLVED When a student mixes 50 mL of 1.0 M HCl and 50 mL of 1.0 M NaOH in a coffee cup Hcl Naoh Calorimetry The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. The hcl and naoh then react until the solution temperature. Obtain four styrofoam cups and two plastic covers. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. 2). Hcl Naoh Calorimetry.
From www.numerade.com
SOLVED 50.0 mL of a solution of HCl is combined with 100.0 mL of 1.05 M NaOH in a calorimeter Hcl Naoh Calorimetry Using a coffee cup calorimeter, the. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which. Hcl Naoh Calorimetry.
From www.numerade.com
SOLVED A coffeecup calorimetry experiment was performed to determine the heat of Hcl Naoh Calorimetry We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. At the instant of mixing, you have 100.0 ml of a mixture of hcl. Hcl Naoh Calorimetry.
From ar.inspiredpencil.com
Calorimetry Hcl Naoh Calorimetry The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. Obtain four styrofoam cups and two plastic covers. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh. Hcl Naoh Calorimetry.
From www.chegg.com
Solved B. Heat of Neutralization of HClNaOH 1. Temp. of Hcl Naoh Calorimetry You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. Using a coffee cup calorimeter, the. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. The hcl and naoh then react until the solution temperature. 2) place 50.0. Hcl Naoh Calorimetry.
From www.studypool.com
SOLUTION Missing values in a calorimetry experiment to determine enthalpy of reaction of hcl Hcl Naoh Calorimetry Using a coffee cup calorimeter, the. The hcl and naoh then react until the solution temperature. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k.. Hcl Naoh Calorimetry.
From www.numerade.com
SOLVED Data Sheet for Hydrochloric Acid Sodium Hydroxide Calorimetry Partner Reaction Reaction Hcl Naoh Calorimetry The hcl and naoh then react until the solution temperature. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. Obtain four styrofoam cups and two plastic covers. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. We used a constructed calorimeter and thermometer. Hcl Naoh Calorimetry.
From mariela-kcarroll.blogspot.com
Titration Curve of Hcl and Naoh Hcl Naoh Calorimetry Using a coffee cup calorimeter, the. Obtain four styrofoam cups and two plastic covers. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. At the instant of mixing, you have 100.0 ml of a mixture. Hcl Naoh Calorimetry.
From www.researchgate.net
(PDF) Specific Heat of Solution of HClNaOH Reaction by a Simple Handmade Calorimeter Hcl Naoh Calorimetry You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Obtain four styrofoam cups and two plastic covers. 2) place 50.0 ml of hcl. Hcl Naoh Calorimetry.
From www.toppr.com
Enthalpy of neutralization of HCl by NaOH is 55.84 kJ/mol and by NH_4OH is 51.34 kJ/mol. The Hcl Naoh Calorimetry The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. Using a coffee cup calorimeter, the. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. You will use either hcl or h 2 so 4 as your acid (you may. Hcl Naoh Calorimetry.
From www.youtube.com
Determination of Heat capacity of Calorimeter & enthalpy of Neutralization of HCl with NaOH Hcl Naoh Calorimetry You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. The hcl and naoh then react until the solution temperature. Using a coffee cup calorimeter, the. We used a. Hcl Naoh Calorimetry.
From www.studypool.com
SOLUTION Missing values in a calorimetry experiment to determine enthalpy of reaction of hcl Hcl Naoh Calorimetry The hcl and naoh then react until the solution temperature. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Obtain four styrofoam cups and two plastic covers. Using a coffee cup calorimeter, the. You will use either hcl or h 2 so 4 as. Hcl Naoh Calorimetry.
From www.youtube.com
Hess's Law Lab Demonstration with NaOH and HCl (Part 2 Data & Calculation) Julia Le YouTube Hcl Naoh Calorimetry The hcl and naoh then react until the solution temperature. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Using a coffee cup calorimeter, the. Obtain four styrofoam cups and two plastic covers. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous. Hcl Naoh Calorimetry.
From oneclass.com
OneClass A student carries out a calorimetry experiment using HCl and NaOH. What effect will Hcl Naoh Calorimetry 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. Obtain four styrofoam cups and two plastic covers. Using a coffee cup calorimeter, the. The hcl and naoh then react until the solution temperature. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. We used. Hcl Naoh Calorimetry.
From www.chegg.com
Solved The heat of neutralization of HCl(aq) and NaOH(aq) is Hcl Naoh Calorimetry At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. The hcl and naoh then react until the solution temperature. We used a constructed calorimeter and thermometer to measure the temperature. Hcl Naoh Calorimetry.
From www.youtube.com
Determination of Heat of Neutralization of a Strong Acid(HCl) with Strong Base(NaOH Hcl Naoh Calorimetry We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Using a coffee cup calorimeter, the. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. 2) place 50.0 ml of hcl and 50.0. Hcl Naoh Calorimetry.
From www.youtube.com
Calorimetry Lab Heat of Solution of NaOH YouTube Hcl Naoh Calorimetry The hcl and naoh then react until the solution temperature. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. 2) place 50.0 ml of hcl and 50.0 ml of. Hcl Naoh Calorimetry.
From www.youtube.com
Heat of neutralisation for the reaction `NaOH+HCl to NaCl+H_(2)O` is 57.1 kJ `mol^(1 YouTube Hcl Naoh Calorimetry At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. Obtain four styrofoam cups and two plastic covers. You will use either hcl or h 2 so 4 as your acid. Hcl Naoh Calorimetry.
From www.numerade.com
SOLVED PART II HEAT OF NEUTRALIZATION OF STRONG ACID, HCL AND STRONG BASE, NAOH Balanced Hcl Naoh Calorimetry You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. Obtain four styrofoam cups and two plastic covers. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh. Hcl Naoh Calorimetry.
From www.coursehero.com
[Solved] Calorimetry Lab Report Sheet Prepare NaOH and HCl Solutions... Course Hero Hcl Naoh Calorimetry You will use either hcl or h 2 so 4 as your acid (you may choose.), and naoh as your base. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. Obtain four styrofoam cups and two plastic covers. The hcl and naoh then react. Hcl Naoh Calorimetry.
From www.chegg.com
Solved Procedure 1. Calorimeter Has o. Procedure 2. NaOH/HCl Hcl Naoh Calorimetry We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. You will use either hcl or h 2 so 4 as your acid (you may choose.),. Hcl Naoh Calorimetry.
From www.chegg.com
Solved Procedure 1. Calorimeter Has o. Procedure 2. NaOH/HCl Hcl Naoh Calorimetry 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. The hcl and naoh then react until the solution temperature. At the instant of mixing, you have 100.0 ml of a. Hcl Naoh Calorimetry.
From slideplayer.com
THERMODYNAMICS Intro & Calorimetry. ppt download Hcl Naoh Calorimetry At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. The hcl and naoh then react until the solution temperature. Obtain four styrofoam cups and two plastic covers. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be. Hcl Naoh Calorimetry.
From selfdirectedce.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Tutorial สรุปเนื้อหาnaoh Hcl Naoh Calorimetry 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. Obtain four styrofoam cups and two plastic covers. The experimental apparatus consists of a calorimeter containing 1.00 m aqueous naoh and a reservoir containing 2.00 m aqueous hcl. The hcl and naoh then react until the solution temperature. Using a coffee cup calorimeter, the. At the. Hcl Naoh Calorimetry.
From www.chegg.com
Solved In a calorimeter 40.0 mL 1.00 M HCl and 40.0 ml NaOH Hcl Naoh Calorimetry At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. The hcl and naoh then react until the solution temperature. Using a coffee cup calorimeter, the.. Hcl Naoh Calorimetry.
From www.numerade.com
Table 11.2 Heat Capacity and Enthalpy Change Run 1 Run 2 Average Initial Temperature of HCl and Hcl Naoh Calorimetry Using a coffee cup calorimeter, the. The hcl and naoh then react until the solution temperature. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. We used a constructed calorimeter and thermometer to measure the temperature of deionized water and the heat capacity which we calculated to be 45 j/k. At the instant of mixing,. Hcl Naoh Calorimetry.
From www.chegg.com
Solved Procedure 1. Calorimeter Has o. Procedure 2. NaOH/HCl Hcl Naoh Calorimetry At the instant of mixing, you have 100.0 ml of a mixture of hcl and naoh at 22.0 °c. 2) place 50.0 ml of hcl and 50.0 ml of naoh in separate graduated. The hcl and naoh then react until the solution temperature. You will use either hcl or h 2 so 4 as your acid (you may choose.), and. Hcl Naoh Calorimetry.