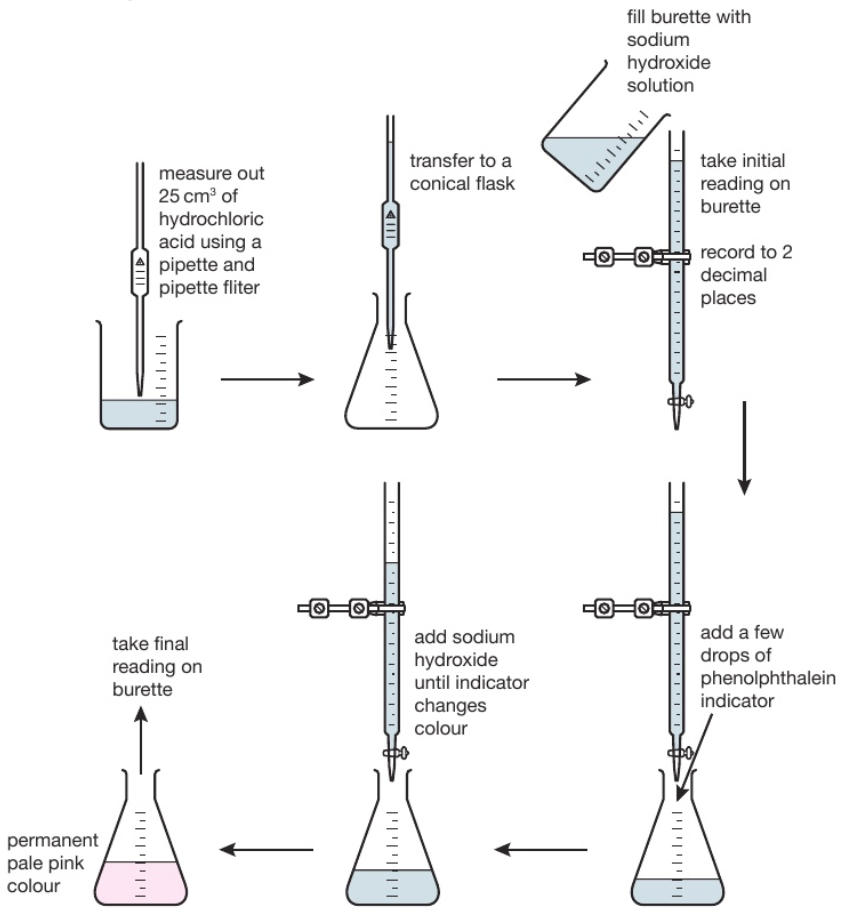
Titration Basic Steps . A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. The steps in a titration reaction are outlined below. We consider it to be analysis because we use it to make a measurement. Step 1 use a small amount of. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Here are steps and the purpose behind each one that will help you achieve an accurate result. Titration is a type of chemical analysis. For example, you can use it to find the concentration of a solution of an acid. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Several drops of an indicator are added to the. The basic process involves adding a standard solution of one reagent to a known amount of the A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
from www.tutormyself.com
Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The steps in a titration reaction are outlined below. For example, you can use it to find the concentration of a solution of an acid. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. We consider it to be analysis because we use it to make a measurement. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Here are steps and the purpose behind each one that will help you achieve an accurate result. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Step 1 use a small amount of.
233 (Triple only) describe how to carry out an acidalkali titration
Titration Basic Steps Here are steps and the purpose behind each one that will help you achieve an accurate result. Titration is a type of chemical analysis. The steps in a titration reaction are outlined below. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Several drops of an indicator are added to the. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Step 1 use a small amount of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The basic process involves adding a standard solution of one reagent to a known amount of the A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For example, you can use it to find the concentration of a solution of an acid. Here are steps and the purpose behind each one that will help you achieve an accurate result. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. We consider it to be analysis because we use it to make a measurement.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Titration Basic Steps A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Here are steps and the purpose behind each one that will help you achieve an accurate result. Titration is a way of measuring the concentration of. Titration Basic Steps.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Basic Steps The basic process involves adding a standard solution of one reagent to a known amount of the A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. Here are steps and the purpose behind each one that will help you achieve an accurate result. We consider it to be analysis because we use it to. Titration Basic Steps.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Basic Steps The basic process involves adding a standard solution of one reagent to a known amount of the Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A titration is a laboratory. Titration Basic Steps.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Basic Steps The steps in a titration reaction are outlined below. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Here are steps and the purpose behind each one that will help you achieve an accurate result. Several drops of an indicator are added to the.. Titration Basic Steps.
From facts.net
8 Captivating Facts About AcidBase Titration Titration Basic Steps Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. Several drops of an indicator are added to the. Titration is a way of measuring the concentration of something, usually the concentration of a substance in. Titration Basic Steps.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Basic Steps A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Step 1 use a small amount of. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. The steps in a titration reaction are outlined. Titration Basic Steps.
From mungfali.com
Acid Base Titration Procedure Titration Basic Steps Here are steps and the purpose behind each one that will help you achieve an accurate result. The basic process involves adding a standard solution of one reagent to a known amount of the Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The steps in a titration reaction are. Titration Basic Steps.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Basic Steps Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Step 1 use a small amount of. The basic process involves adding a standard solution of one reagent to a known amount of the We consider it to be analysis because we use it to make a. Titration Basic Steps.
From mungfali.com
Titration Steps Titration Basic Steps Several drops of an indicator are added to the. The steps in a titration reaction are outlined below. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Here are steps and the purpose behind each one that will help you achieve an accurate result. A titration is a laboratory technique. Titration Basic Steps.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Titration Basic Steps A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Here are steps and the purpose behind each one that will help you achieve an accurate result. The basic process involves adding a standard solution of one reagent to a known amount of the Titration is a type of chemical. Titration Basic Steps.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Basic Steps The basic process involves adding a standard solution of one reagent to a known amount of the Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions. Titration Basic Steps.
From www.dreamstime.com
Phenolphthalein Indicator in Acidbase Titration Stock Vector Titration Basic Steps Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Here are steps and the purpose behind each one that will help you achieve an accurate result. The steps in a titration reaction are outlined below. Titration is the slow addition of one solution of a known concentration (called a titrant). Titration Basic Steps.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Basic Steps Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. Titration is a. Titration Basic Steps.
From mungfali.com
Acid Base Titration Experiment Titration Basic Steps Here are steps and the purpose behind each one that will help you achieve an accurate result. For example, you can use it to find the concentration of a solution of an acid. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Step 1 use a small amount of. A. Titration Basic Steps.
From www.science-revision.co.uk
Titrations Titration Basic Steps Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is a type of chemical analysis. For example, you can use it to find the concentration of a solution of an acid. Step 1 use a small amount of. Here are steps and the purpose behind. Titration Basic Steps.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration Basic Steps In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. For example, you can use it to find the concentration of a solution of an acid. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration Basic Steps.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Titration Basic Steps Here are steps and the purpose behind each one that will help you achieve an accurate result. The steps in a titration reaction are outlined below. The basic process involves adding a standard solution of one reagent to a known amount of the We consider it to be analysis because we use it to make a measurement. Titration is a. Titration Basic Steps.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Titration Basic Steps Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Here are steps and the purpose behind each one that will help you achieve an accurate result. Several drops of an indicator are added to the. We consider it to be analysis because we use it to. Titration Basic Steps.
From www.vrogue.co
Wcln Precipitation Titration Calculations Chemistry Y vrogue.co Titration Basic Steps Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The steps in a titration reaction are outlined below. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A measured volume of an acid of unknown concentration is added to. Titration Basic Steps.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Basic Steps Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. The basic process involves adding a standard solution of one reagent to a known amount of the Several drops of an indicator are added to the. The steps in a titration reaction are outlined below. A measured volume of an acid. Titration Basic Steps.
From www.slideserve.com
PPT ENZYME CATALYSIS LAB PowerPoint Presentation, free download ID Titration Basic Steps In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. The basic process involves adding a standard solution of one reagent to a known amount of the Titration is a procedure used in chemistry in order to determine the molarity of an acid or a. Titration Basic Steps.
From tuitiontube.com
Details about AcidBase Titration Lab in Easy Language Tuition Tube Titration Basic Steps In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. Here. Titration Basic Steps.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Basic Steps Step 1 use a small amount of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For example, you can use it to find the concentration of a solution of an acid. A measured volume of an acid of unknown concentration is added to an erlenmeyer. Titration Basic Steps.
From www.savemyexams.co.uk
Titrations (1.2.9) DP IB Chemistry SL Revision Notes 2016 Save My Titration Basic Steps A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. We consider it to be analysis because we use it to make a measurement. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The basic process. Titration Basic Steps.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Basic Steps In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. The steps in a titration reaction are outlined below. Several drops of an indicator are added to the. Step 1 use a small amount of. The basic process involves adding a standard solution of one. Titration Basic Steps.
From www.slideserve.com
PPT AcidBase Titration and pH PowerPoint Presentation, free download Titration Basic Steps Titration is a type of chemical analysis. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Here are steps and the purpose behind each one that will help you achieve an accurate result. Step 1 use a small amount of. Several drops of an indicator are. Titration Basic Steps.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Basic Steps Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For example, you can use it to find the concentration of a solution of an acid. We consider it to be analysis because we use it to make a measurement. Step 1 use a small amount of.. Titration Basic Steps.
From www.youtube.com
Titration How to perform Titration Experiment/Practical YouTube Titration Basic Steps The steps in a titration reaction are outlined below. Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. Step 1 use a small amount of.. Titration Basic Steps.
From mungfali.com
Titration Steps Titration Basic Steps For example, you can use it to find the concentration of a solution of an acid. Here are steps and the purpose behind each one that will help you achieve an accurate result. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a procedure used in chemistry. Titration Basic Steps.
From www.thinkswap.com
Titration Practical Chemistry Year 12 SACE Thinkswap Titration Basic Steps In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. The basic process involves adding a standard solution of one reagent to a known amount of the For example, you can use it to find the concentration of a solution of an acid. Titration is. Titration Basic Steps.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Basic Steps We consider it to be analysis because we use it to make a measurement. The steps in a titration reaction are outlined below. Step 1 use a small amount of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. Titration is a way of measuring the. Titration Basic Steps.
From ar.inspiredpencil.com
Titration Experiment Using Phenolphthalein Titration Basic Steps A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a type of chemical analysis. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. The basic process involves adding a standard solution of one. Titration Basic Steps.
From www.learnsci.com
LearnSci LabSim Titration End Point Titration Basic Steps For example, you can use it to find the concentration of a solution of an acid. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. A measured volume of an acid of unknown concentration is added to an erlenmeyer flask. A titration is a laboratory technique. Titration Basic Steps.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Basic Steps Titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. Here are steps and the purpose behind each one that will help you achieve an accurate result. We consider it to be analysis because we use it to make a measurement. Titration is a way of measuring the concentration of something,. Titration Basic Steps.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Basic Steps The steps in a titration reaction are outlined below. In this tutorial, you will find information on titration, including the chemicals that are commonly used and the chemical reactions that make titration work, as. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a type of chemical. Titration Basic Steps.