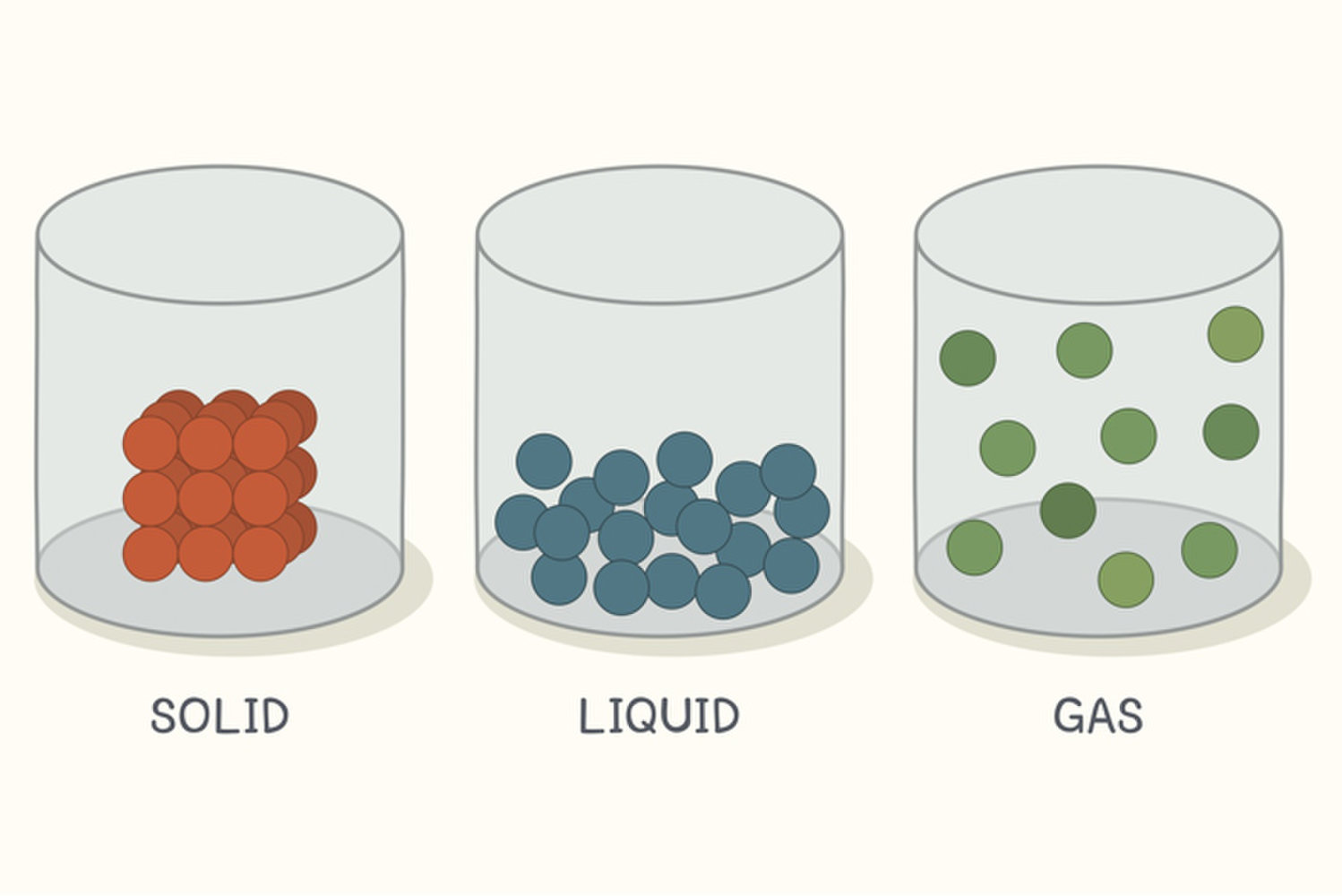
Why Do Solids Liquids And Gases Have Different Properties Class 6 . In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Solids, liquids and gases have different properties. This model explains the properties of substances in. The three states of matter can be represented by the particle model. In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place with each other. Difference between solid liquid and gases. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. A solid has a fixed shape and volume but some solids can change shape when a force is applied. As the temperature of a gas increases, what happens to the particles of. Gases, on the other hand, have uniquely different properties compared to solids and liquids. Why do liquids and gases take the shape of their containers?
from socratic.org
In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place with each other. Why do liquids and gases take the shape of their containers? A solid has a fixed shape and volume but some solids can change shape when a force is applied. The three states of matter can be represented by the particle model. This model explains the properties of substances in. Difference between solid liquid and gases. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Solids, liquids and gases have different properties. Gases, on the other hand, have uniquely different properties compared to solids and liquids.
What are examples of gases, liquids, and solids? Socratic
Why Do Solids Liquids And Gases Have Different Properties Class 6 Why do liquids and gases take the shape of their containers? The three states of matter can be represented by the particle model. A solid has a fixed shape and volume but some solids can change shape when a force is applied. Gases, on the other hand, have uniquely different properties compared to solids and liquids. Difference between solid liquid and gases. As the temperature of a gas increases, what happens to the particles of. Solids, liquids and gases have different properties. In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place with each other. This model explains the properties of substances in. Why do liquids and gases take the shape of their containers? In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating.
From exyfeersx.blob.core.windows.net
Why Solid Liquid And Gas Have Different Properties at Helen Gatlin blog Why Do Solids Liquids And Gases Have Different Properties Class 6 Solids, liquids and gases have different properties. Gases, on the other hand, have uniquely different properties compared to solids and liquids. This model explains the properties of substances in. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. In this tutorial, you will learn about the properties of the solid,. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From exyfeersx.blob.core.windows.net
Why Solid Liquid And Gas Have Different Properties at Helen Gatlin blog Why Do Solids Liquids And Gases Have Different Properties Class 6 Why do liquids and gases take the shape of their containers? As the temperature of a gas increases, what happens to the particles of. This model explains the properties of substances in. Solids, liquids and gases have different properties. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. The three. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.snexplores.org
Explainer What are the different states of matter? Why Do Solids Liquids And Gases Have Different Properties Class 6 Why do liquids and gases take the shape of their containers? Gases, on the other hand, have uniquely different properties compared to solids and liquids. Difference between solid liquid and gases. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. A solid has a fixed shape and volume but some solids. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Why Do Solids Liquids And Gases Have Different Properties Class 6 A solid has a fixed shape and volume but some solids can change shape when a force is applied. The three states of matter can be represented by the particle model. Gases, on the other hand, have uniquely different properties compared to solids and liquids. Why do liquids and gases take the shape of their containers? In this tutorial, you. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From dxojwesjc.blob.core.windows.net
Lesson Plan Properties Of Solids Liquids And Gases at Albert Mitchell blog Why Do Solids Liquids And Gases Have Different Properties Class 6 Difference between solid liquid and gases. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Why do liquids and gases take the shape of their containers? In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place with each other.. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.pinterest.com
10 Examples of Solids, Liquids, Gases, and Plasma Solid liquid gas Why Do Solids Liquids And Gases Have Different Properties Class 6 In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. A solid has a fixed shape and volume but some solids can change shape when a force is applied. Solids, liquids and gases have different properties. Difference between solid liquid and gases. The three states of matter can be represented by the. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From diagramlibraryconjoin.z19.web.core.windows.net
Solid Liquid And Gas Diagram Why Do Solids Liquids And Gases Have Different Properties Class 6 This model explains the properties of substances in. A solid has a fixed shape and volume but some solids can change shape when a force is applied. As the temperature of a gas increases, what happens to the particles of. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Solids,. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii Why Do Solids Liquids And Gases Have Different Properties Class 6 In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. This model explains the properties of substances in. Difference between solid liquid and gases. Gases, on the other hand, have uniquely different properties compared to solids and liquids. In solids, the atoms and molecules that make up the object/matter have low. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Why Do Solids Liquids And Gases Have Different Properties Class 6 In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Difference between solid liquid and gases. Gases, on the other hand, have uniquely different properties compared to solids and liquids. Solids, liquids and gases have different properties. As the temperature of a gas increases, what happens to the particles of. A solid. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From brainly.in
why solid liquid and gases have different properties Brainly.in Why Do Solids Liquids And Gases Have Different Properties Class 6 This model explains the properties of substances in. Difference between solid liquid and gases. Solids, liquids and gases have different properties. The three states of matter can be represented by the particle model. In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place with each other. Gases, on. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.youtube.com
Difference Between Solid Liquid And Gases State Of Matter Why Do Solids Liquids And Gases Have Different Properties Class 6 In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Difference between solid liquid and gases. Gases, on the other hand, have uniquely different properties compared to solids and liquids. As the temperature. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.pinterest.ph
Properties of Solids, Liquids, Gases Compared Teachoo Science Why Do Solids Liquids And Gases Have Different Properties Class 6 As the temperature of a gas increases, what happens to the particles of. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. A solid has a fixed shape and volume but some solids can change shape when a force is applied. The three states of matter can be represented by. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.slideserve.com
PPT Solids,gases,Liquids PowerPoint Presentation, free download ID Why Do Solids Liquids And Gases Have Different Properties Class 6 In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Difference between solid liquid and gases. Gases, on the other hand, have uniquely different properties compared to solids and liquids. This model explains the properties of substances in. In solids, the atoms and molecules that make up the object/matter have low. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas Why Do Solids Liquids And Gases Have Different Properties Class 6 Gases, on the other hand, have uniquely different properties compared to solids and liquids. This model explains the properties of substances in. A solid has a fixed shape and volume but some solids can change shape when a force is applied. The three states of matter can be represented by the particle model. As the temperature of a gas increases,. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From joisrkcqc.blob.core.windows.net
Which Properties Do All Solids Liquids And Gases Have at Estelle Lewis blog Why Do Solids Liquids And Gases Have Different Properties Class 6 This model explains the properties of substances in. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Why do liquids and gases take the shape of their containers? As the temperature of a gas increases, what happens to the particles of. The three states of matter can be represented by. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From joisrkcqc.blob.core.windows.net
Which Properties Do All Solids Liquids And Gases Have at Estelle Lewis blog Why Do Solids Liquids And Gases Have Different Properties Class 6 Difference between solid liquid and gases. The three states of matter can be represented by the particle model. Solids, liquids and gases have different properties. As the temperature of a gas increases, what happens to the particles of. In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.youtube.com
Properties of Solids, Liquids and Gases YouTube Why Do Solids Liquids And Gases Have Different Properties Class 6 In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. A solid has a fixed shape and volume but some solids can change shape when a force is applied. Gases, on the other hand, have uniquely different properties compared to solids and liquids. As the temperature of a gas increases, what happens. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Why Do Solids Liquids And Gases Have Different Properties Class 6 Why do liquids and gases take the shape of their containers? Difference between solid liquid and gases. A solid has a fixed shape and volume but some solids can change shape when a force is applied. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. The three states of matter. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From exyfeersx.blob.core.windows.net
Why Solid Liquid And Gas Have Different Properties at Helen Gatlin blog Why Do Solids Liquids And Gases Have Different Properties Class 6 This model explains the properties of substances in. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Solids, liquids and gases have different properties. The three states of matter can be represented. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From exyfeersx.blob.core.windows.net
Why Solid Liquid And Gas Have Different Properties at Helen Gatlin blog Why Do Solids Liquids And Gases Have Different Properties Class 6 Why do liquids and gases take the shape of their containers? The three states of matter can be represented by the particle model. As the temperature of a gas increases, what happens to the particles of. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Solids, liquids and gases have. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.youtube.com
States of matter 🚗💧☁️ Solid, Liquid & Gas Learn with examples YouTube Why Do Solids Liquids And Gases Have Different Properties Class 6 A solid has a fixed shape and volume but some solids can change shape when a force is applied. The three states of matter can be represented by the particle model. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Difference between solid liquid and gases. In solids, the atoms. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.slideshare.net
(Lesson 2)properties of solids, liquids, and gases Why Do Solids Liquids And Gases Have Different Properties Class 6 Gases, on the other hand, have uniquely different properties compared to solids and liquids. Solids, liquids and gases have different properties. The three states of matter can be represented by the particle model. As the temperature of a gas increases, what happens to the particles of. Difference between solid liquid and gases. Why do liquids and gases take the shape. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From slgas03.blogspot.com
solid,liquid and gas States of Matter Why Do Solids Liquids And Gases Have Different Properties Class 6 Why do liquids and gases take the shape of their containers? In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place with each other. As the temperature of a gas increases, what happens to the particles of. In this tutorial, you will learn about the properties of the. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.slideshare.net
solids, liquids and gases Why Do Solids Liquids And Gases Have Different Properties Class 6 As the temperature of a gas increases, what happens to the particles of. Gases, on the other hand, have uniquely different properties compared to solids and liquids. The three states of matter can be represented by the particle model. Solids, liquids and gases have different properties. In a solid like this brick, the particles are regularly arranged touching their neighbours. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different Why Do Solids Liquids And Gases Have Different Properties Class 6 In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place with each other. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. As the temperature of a gas increases, what happens to the particles of. Gases, on the other. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From joicpveqm.blob.core.windows.net
Solids Liquids Gases Homework at Kimberly Terrell blog Why Do Solids Liquids And Gases Have Different Properties Class 6 Gases, on the other hand, have uniquely different properties compared to solids and liquids. The three states of matter can be represented by the particle model. This model explains the properties of substances in. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. In this tutorial, you will learn about. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.slideshare.net
Unit 1 Notes Why Do Solids Liquids And Gases Have Different Properties Class 6 This model explains the properties of substances in. A solid has a fixed shape and volume but some solids can change shape when a force is applied. Solids, liquids and gases have different properties. As the temperature of a gas increases, what happens to the particles of. Why do liquids and gases take the shape of their containers? In this. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.primaryworks.co.uk
PowerPoint KS2 explanation on solids, liquids & gases KS2 primary Why Do Solids Liquids And Gases Have Different Properties Class 6 As the temperature of a gas increases, what happens to the particles of. The three states of matter can be represented by the particle model. In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place with each other. Gases, on the other hand, have uniquely different properties compared. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From sciencenotes.org
States of Matter Why Do Solids Liquids And Gases Have Different Properties Class 6 Difference between solid liquid and gases. As the temperature of a gas increases, what happens to the particles of. Solids, liquids and gases have different properties. In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. The three states of matter can be represented by the particle model. A solid has a. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.britannica.com
solid Definition & Facts Britannica Why Do Solids Liquids And Gases Have Different Properties Class 6 Difference between solid liquid and gases. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. As the temperature of a gas increases, what happens to the particles of. The three states of matter can be represented by the particle model. Gases, on the other hand, have uniquely different properties compared. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From www.slideserve.com
PPT Solids,gases,Liquids PowerPoint Presentation, free download ID Why Do Solids Liquids And Gases Have Different Properties Class 6 In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. Solids, liquids and gases have different properties. In solids, the atoms and molecules that make up the object/matter have low energy levels, and are generally locked rigidly into place with each other. In this tutorial, you will learn about the properties. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison Why Do Solids Liquids And Gases Have Different Properties Class 6 Gases, on the other hand, have uniquely different properties compared to solids and liquids. In a solid like this brick, the particles are regularly arranged touching their neighbours and move only by vibrating. A solid has a fixed shape and volume but some solids can change shape when a force is applied. The three states of matter can be represented. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From socratic.org
What are examples of gases, liquids, and solids? Socratic Why Do Solids Liquids And Gases Have Different Properties Class 6 Difference between solid liquid and gases. Gases, on the other hand, have uniquely different properties compared to solids and liquids. As the temperature of a gas increases, what happens to the particles of. This model explains the properties of substances in. Solids, liquids and gases have different properties. In this tutorial, you will learn about the properties of the solid,. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From klabezmmm.blob.core.windows.net
Solids Liquids And Gases Have Different Observable Properties And Why Do Solids Liquids And Gases Have Different Properties Class 6 A solid has a fixed shape and volume but some solids can change shape when a force is applied. Gases, on the other hand, have uniquely different properties compared to solids and liquids. As the temperature of a gas increases, what happens to the particles of. In this tutorial, you will learn about the properties of the solid, liquid, and. Why Do Solids Liquids And Gases Have Different Properties Class 6.
From joihbnecv.blob.core.windows.net
What Is Solid Liquid at Sheila Trotter blog Why Do Solids Liquids And Gases Have Different Properties Class 6 Solids, liquids and gases have different properties. Why do liquids and gases take the shape of their containers? In this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. A solid has a fixed shape and volume but some solids can change shape when a force is applied. Gases, on the other hand,. Why Do Solids Liquids And Gases Have Different Properties Class 6.