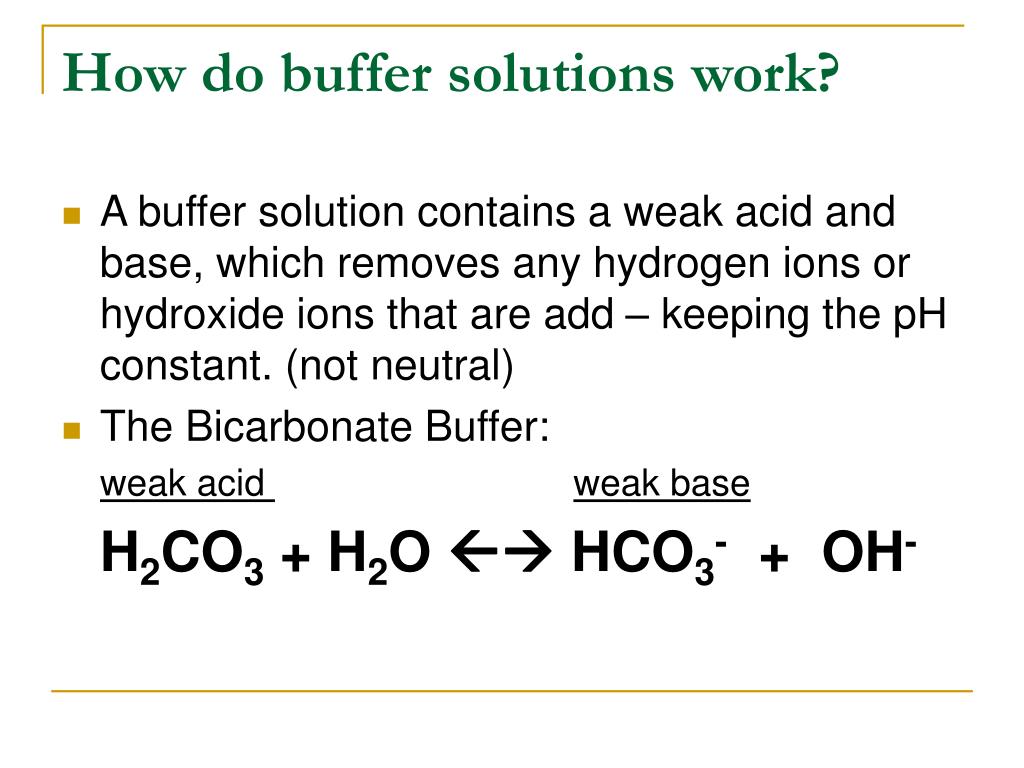
Buffer Solution Summary . A solution containing a mixture of an acid and its conjugate base, or of a base and its. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. Either a weak acid plus a salt derived from that weak acid, or a weak. It is able to neutralize small amounts. what is a buffer solution? a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant.
from www.slideserve.com
Either a weak acid plus a salt derived from that weak acid, or a weak. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: what is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small amounts. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. A solution containing a mixture of an acid and its conjugate base, or of a base and its.
PPT Buffers PowerPoint Presentation, free download ID5687114
Buffer Solution Summary a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution containing a mixture of an acid and its conjugate base, or of a base and its. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Either a weak acid plus a salt derived from that weak acid, or a weak. a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small amounts. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is a buffer solution? buffers resist dramatic changes in ph by being composed of certain pairs of solutes:
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer Solution Summary what is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Either a weak acid plus a salt derived from that weak acid, or a weak. A solution containing a mixture of an acid and its conjugate base, or of a base and its. A. Buffer Solution Summary.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1031156 Buffer Solution Summary A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A solution containing a mixture of an acid and its conjugate base, or of a base and its. a buffer solution is a solution where the ph does not change significantly on dilution or if an. Buffer Solution Summary.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution Summary A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. what is a buffer solution? a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution containing a mixture of an acid and its conjugate. Buffer Solution Summary.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution Summary what is a buffer solution? a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. buffers resist dramatic changes. Buffer Solution Summary.
From www.slideserve.com
PPT BUFFER SOLUTION PowerPoint Presentation, free download ID6580038 Buffer Solution Summary a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. Either a weak acid plus a salt derived from that weak. Buffer Solution Summary.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer Solution Summary A solution containing a mixture of an acid and its conjugate base, or of a base and its. It is able to neutralize small amounts. a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. a buffer is a solution that can resist ph change upon. Buffer Solution Summary.
From www.pinterest.es
Understanding Buffer Solution Chemistry A Complete Guide Buffer Solution Summary buffers resist dramatic changes in ph by being composed of certain pairs of solutes: a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base. Buffer Solution Summary.
From www.slideserve.com
PPT Hydrolysis of Salts and pH of Buffer Solutions PowerPoint Buffer Solution Summary a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution containing a mixture of an acid and its conjugate base, or of a base and its. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to. Buffer Solution Summary.
From www.ck12.org
Buffer Solutions Example 2 ( Video ) Chemistry CK12 Foundation Buffer Solution Summary a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer solution is a solution where the ph does not change significantly on dilution or. Buffer Solution Summary.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID2813415 Buffer Solution Summary Either a weak acid plus a salt derived from that weak acid, or a weak. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. It is able to neutralize small amounts. a buffer is an aqueous solution containing a weak acid and. Buffer Solution Summary.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co Buffer Solution Summary A solution containing a mixture of an acid and its conjugate base, or of a base and its. It is able to neutralize small amounts. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is a solution that can resist ph change upon. Buffer Solution Summary.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution Summary a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak. A buffer solution is one which resists. Buffer Solution Summary.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Buffer Solution Summary It is able to neutralize small amounts. A solution containing a mixture of an acid and its conjugate base, or of a base and its. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak. a buffer is an. Buffer Solution Summary.
From www.slideserve.com
PPT Buffer solutions PowerPoint Presentation, free download ID6578454 Buffer Solution Summary what is a buffer solution? a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is an. Buffer Solution Summary.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Summary a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small amounts. Either a weak acid plus a salt. Buffer Solution Summary.
From www.slideserve.com
PPT Buffer Solutions, Function PowerPoint Presentation, free download Buffer Solution Summary a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. Either a weak acid plus a salt derived from that weak acid, or a weak. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: A solution containing a mixture of an. Buffer Solution Summary.
From www.slideshare.net
Summary of buffer solution Buffer Solution Summary Either a weak acid plus a salt derived from that weak acid, or a weak. a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is. Buffer Solution Summary.
From www.slideserve.com
PPT Buffer Solutions and pH Resistance PowerPoint Presentation, free Buffer Solution Summary Either a weak acid plus a salt derived from that weak acid, or a weak. what is a buffer solution? It is able to neutralize small amounts. A solution containing a mixture of an acid and its conjugate base, or of a base and its. a buffer solution is a solution where the ph does not change significantly. Buffer Solution Summary.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses Buffer Solution Summary A solution containing a mixture of an acid and its conjugate base, or of a base and its. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. a buffer is an aqueous solution containing a weak acid and its conjugate base or. Buffer Solution Summary.
From www.slideserve.com
PPT CHEMISTRY Chapter 15 Applications of Aqueous Equilibria Buffer Solution Summary what is a buffer solution? a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. A solution containing a mixture. Buffer Solution Summary.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Buffer Solution Summary a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. It is able to neutralize small amounts. a buffer is. Buffer Solution Summary.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 Buffer Solution Summary Either a weak acid plus a salt derived from that weak acid, or a weak. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution containing a mixture of an acid and its conjugate base, or of a base and its. a buffer solution is a solution. Buffer Solution Summary.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk Buffer Solution Summary what is a buffer solution? It is able to neutralize small amounts. A solution containing a mixture of an acid and its conjugate base, or of a base and its. Either a weak acid plus a salt derived from that weak acid, or a weak. buffers resist dramatic changes in ph by being composed of certain pairs of. Buffer Solution Summary.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution Buffer Solution Summary a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Either a weak acid plus a salt derived from that weak acid, or a weak. a. Buffer Solution Summary.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID7055849 Buffer Solution Summary A solution containing a mixture of an acid and its conjugate base, or of a base and its. a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. It is able to neutralize small amounts. what is a buffer solution? A buffer solution is one which. Buffer Solution Summary.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Buffer Solution Summary buffers resist dramatic changes in ph by being composed of certain pairs of solutes: Either a weak acid plus a salt derived from that weak acid, or a weak. what is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A. Buffer Solution Summary.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Buffer Solution Summary buffers resist dramatic changes in ph by being composed of certain pairs of solutes: a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. Either a weak acid plus a salt derived from that weak acid, or a weak. A solution containing a mixture of an. Buffer Solution Summary.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download Buffer Solution Summary It is able to neutralize small amounts. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. buffers resist dramatic changes in ph by being composed of certain pairs of solutes: a buffer solution is a solution where the ph does not change significantly on dilution or if. Buffer Solution Summary.
From www.ck12.org
Buffer Solutions Example 1 ( Video ) Chemistry CK12 Foundation Buffer Solution Summary Either a weak acid plus a salt derived from that weak acid, or a weak. It is able to neutralize small amounts. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer solution is a solution where the ph does not change significantly on. Buffer Solution Summary.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Buffer Solution Summary A solution containing a mixture of an acid and its conjugate base, or of a base and its. a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali. Buffer Solution Summary.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID3197944 Buffer Solution Summary It is able to neutralize small amounts. what is a buffer solution? A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. a buffer is an aqueous solution containing a weak acid and its conjugate base or a weak base and its conjugate acid. Either. Buffer Solution Summary.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Buffer Solution Summary Either a weak acid plus a salt derived from that weak acid, or a weak. a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is. Buffer Solution Summary.
From skc13chem.blogspot.com
SKC year 13 Chemistry Buffer solutions Buffer Solution Summary Either a weak acid plus a salt derived from that weak acid, or a weak. A solution containing a mixture of an acid and its conjugate base, or of a base and its. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. . Buffer Solution Summary.
From www.slideserve.com
PPT Buffered Solutions PowerPoint Presentation, free download ID Buffer Solution Summary a buffer is a solution that can resist ph change upon the addition of an acidic or basic components. what is a buffer solution? a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. It is able to neutralize small amounts. A. Buffer Solution Summary.
From www.slideserve.com
PPT Buffer Capacity Lab PowerPoint Presentation, free download ID Buffer Solution Summary Either a weak acid plus a salt derived from that weak acid, or a weak. a buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant. It is able to neutralize small amounts. a buffer is a solution that can resist ph change upon. Buffer Solution Summary.