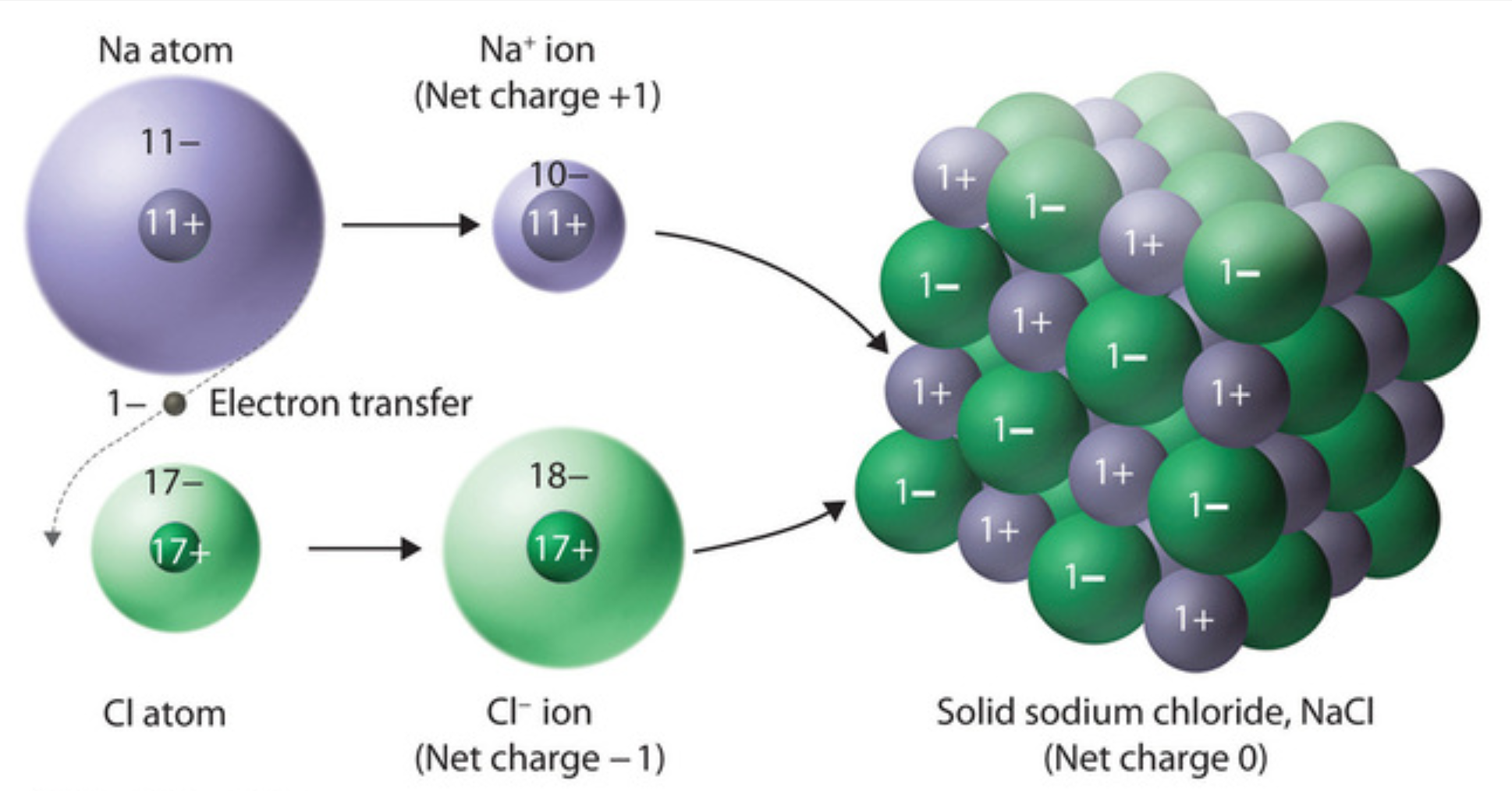
Formation Of Table Salt Equation . the formula for table salt is nacl. the molecular formula of table salt—sodium chloride—is nacl. this reaction is represented as: 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. It is an ionic compound which consists of a chloride. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. Both methods result in the formation of sodium chloride, a vital. revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. 2na (s) + cl₂ (g) → 2nacl (s). In the solid lattice, each ion is surrounded by six ions. sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone on the planet.
from chem.libretexts.org
thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. It is an ionic compound which consists of a chloride. Both methods result in the formation of sodium chloride, a vital. 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. In the solid lattice, each ion is surrounded by six ions. the molecular formula of table salt—sodium chloride—is nacl. Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. 2na (s) + cl₂ (g) → 2nacl (s). sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone on the planet.
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts
Formation Of Table Salt Equation revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Both methods result in the formation of sodium chloride, a vital. sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone on the planet. the molecular formula of table salt—sodium chloride—is nacl. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. It is an ionic compound which consists of a chloride. 2na (s) + cl₂ (g) → 2nacl (s). In the solid lattice, each ion is surrounded by six ions. Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. this reaction is represented as: 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride the formula for table salt is nacl.
From exomrqhri.blob.core.windows.net
Chemical Reaction For Table Salt at Amy French blog Formation Of Table Salt Equation It is an ionic compound which consists of a chloride. 2na (s) + cl₂ (g) → 2nacl (s). In the solid lattice, each ion is surrounded by six ions. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. sodium and chlorine react to form sodium chloride, also known. Formation Of Table Salt Equation.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Formation Of Table Salt Equation the formula for table salt is nacl. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. the molecular formula of table salt—sodium chloride—is nacl. revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. . Formation Of Table Salt Equation.
From exomdamui.blob.core.windows.net
Table Salt Atomic Formula at Pete Alvarez blog Formation Of Table Salt Equation Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. the molecular formula of table salt—sodium chloride—is nacl. 2na (s) + cl₂ (g) → 2nacl (s). the formula for table salt is nacl. sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which. Formation Of Table Salt Equation.
From www.dreamstime.com
Sodium chloride solution stock vector. Illustration of hydrogen 80714673 Formation Of Table Salt Equation the formula for table salt is nacl. Both methods result in the formation of sodium chloride, a vital. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my. Formation Of Table Salt Equation.
From studylib.net
LAB The Synthesis of Table Salt Formation Of Table Salt Equation In the solid lattice, each ion is surrounded by six ions. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. Both methods result in the formation of sodium chloride, a vital. this reaction is represented as: 2na (s) + cl₂ (g) → 2nacl (s). the molecular formula. Formation Of Table Salt Equation.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Formation Of Table Salt Equation Both methods result in the formation of sodium chloride, a vital. In the solid lattice, each ion is surrounded by six ions. revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride the formula for. Formation Of Table Salt Equation.
From riversfansite.blogspot.com
Table Salt Chemical Formula Sodium chloride , commonly known as salt Formation Of Table Salt Equation It is an ionic compound which consists of a chloride. Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. the formula for table salt is nacl. In the solid lattice, each ion is surrounded by six ions. revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written. Formation Of Table Salt Equation.
From awesomehome.co
Is Table Salt A Compound Awesome Home Formation Of Table Salt Equation Both methods result in the formation of sodium chloride, a vital. 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. the molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is surrounded by six ions.. Formation Of Table Salt Equation.
From chemisfast.blogspot.com
Double saltsdefinitionexamples and properties in coordination Formation Of Table Salt Equation Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. this reaction is represented as: It is an ionic compound which consists of a chloride. 2na (s) + cl₂ (g) → 2nacl (s). thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from. Formation Of Table Salt Equation.
From www.alamy.com
an equation (chemistry) showing dissoatiation of table salt in water Formation Of Table Salt Equation this reaction is represented as: sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone on the planet. In the solid lattice, each ion is surrounded by six ions. It is an ionic compound which consists of a chloride. Both methods result in the formation of. Formation Of Table Salt Equation.
From www.youtube.com
Types of Salts Normal or Neutral Salts and Acidic Salts Chemistry Class Formation Of Table Salt Equation thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. Both methods result in the formation of sodium chloride, a vital. revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. sodium and chlorine react to form. Formation Of Table Salt Equation.
From awesomehome.co
Is Table Salt A Compound Awesome Home Formation Of Table Salt Equation Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone. Formation Of Table Salt Equation.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube Formation Of Table Salt Equation the molecular formula of table salt—sodium chloride—is nacl. this reaction is represented as: the formula for table salt is nacl. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. Both methods result in the formation of sodium chloride, a vital. In the solid lattice, each ion. Formation Of Table Salt Equation.
From www.youtube.com
Hydrolysis of Salts YouTube Formation Of Table Salt Equation Both methods result in the formation of sodium chloride, a vital. revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride In the solid lattice, each ion is surrounded by six ions. 2na (s) + cl₂. Formation Of Table Salt Equation.
From cabinet.matttroy.net
Table Salt Chemical Formula Matttroy Formation Of Table Salt Equation this reaction is represented as: Both methods result in the formation of sodium chloride, a vital. sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone on the planet. Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass.. Formation Of Table Salt Equation.
From www.slideshare.net
Gcse c6 science making useful salts Formation Of Table Salt Equation In the solid lattice, each ion is surrounded by six ions. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. the formula for table salt is nacl. revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my. Formation Of Table Salt Equation.
From www.thoughtco.com
Neutralization and Hydrolysis in Salt Formation Formation Of Table Salt Equation the molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is surrounded by six ions. this reaction is represented as: Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. the formula for table salt is nacl. revision notes on preparing soluble salts for the. Formation Of Table Salt Equation.
From www.youtube.com
Making Salts From Acids & Metal Carbonates (GCSE Chemistry) YouTube Formation Of Table Salt Equation 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. revision notes on preparing soluble salts for the cie igcse. Formation Of Table Salt Equation.
From www.thoughtco.com
Chemical Composition of Table Salt Formation Of Table Salt Equation the formula for table salt is nacl. Both methods result in the formation of sodium chloride, a vital. 2na (s) + cl₂ (g) → 2nacl (s). revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the molecular formula of table salt—sodium chloride—is nacl. In the. Formation Of Table Salt Equation.
From stock.adobe.com
Sodium chloride (table salt), chemical structure. Skeletal formula Formation Of Table Salt Equation Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride the formula for table salt is nacl. It is an ionic compound which consists of a chloride. 2na (s) + cl₂ (g) → 2nacl (s). In the solid lattice, each. Formation Of Table Salt Equation.
From www.thoughtco.com
Chemical Composition of Table Salt Formation Of Table Salt Equation It is an ionic compound which consists of a chloride. this reaction is represented as: thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. the formula for table salt is nacl. 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride sodium and chlorine. Formation Of Table Salt Equation.
From stock.adobe.com
Vetor de Neutralization reaction. Acid, base, alkali, salt water Formation Of Table Salt Equation Both methods result in the formation of sodium chloride, a vital. the formula for table salt is nacl. Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. In the solid lattice,. Formation Of Table Salt Equation.
From www.teachoo.com
Salts and it's Properties (with Examples) Acids, Bases and Salt Formation Of Table Salt Equation the molecular formula of table salt—sodium chloride—is nacl. It is an ionic compound which consists of a chloride. sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone on the planet. In the solid lattice, each ion is surrounded by six ions. revision notes on. Formation Of Table Salt Equation.
From chemisfast.blogspot.com
Double saltsdefinitionexamples and properties in coordination Formation Of Table Salt Equation Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. Both methods result in the formation of sodium chloride, a vital. In the solid lattice, each ion is surrounded by six ions. sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to. Formation Of Table Salt Equation.
From exoilfotb.blob.core.windows.net
What Is Table Salt In Chemistry at Christina Stotts blog Formation Of Table Salt Equation 2na (s) + cl₂ (g) → 2nacl (s). sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone on the planet. Both methods result in the formation of sodium chloride, a vital. 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride Nacl is the. Formation Of Table Salt Equation.
From www.alamy.com
Sodium chloride (table salt), chemical structure. Blue skeletal formula Formation Of Table Salt Equation 2na (s) + cl₂ (g) → 2nacl (s). the molecular formula of table salt—sodium chloride—is nacl. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. It is an ionic compound which. Formation Of Table Salt Equation.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Formation Of Table Salt Equation 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. the formula for table salt is nacl. Both methods result in the formation of sodium chloride, a vital. Nacl is the molecular formula of sodium. Formation Of Table Salt Equation.
From elchoroukhost.net
Table Salt Chemical Compound Formula Elcho Table Formation Of Table Salt Equation this reaction is represented as: Both methods result in the formation of sodium chloride, a vital. 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride In the solid lattice, each ion is surrounded by six ions. Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. revision notes. Formation Of Table Salt Equation.
From elchoroukhost.net
What Is The Chemical Formula For Table Salt Elcho Table Formation Of Table Salt Equation the molecular formula of table salt—sodium chloride—is nacl. Both methods result in the formation of sodium chloride, a vital. the formula for table salt is nacl. It is an ionic compound which consists of a chloride. this reaction is represented as: revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the. Formation Of Table Salt Equation.
From www.chegg.com
Solved 2. Table salt has a molecular formula of NaCl (i.e. 1 Formation Of Table Salt Equation thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. this reaction is represented as: Both methods result in the formation of sodium chloride, a vital. It is an ionic compound which consists of a chloride. 2na (s) + cl₂ (g) → 2nacl (s). In the solid lattice, each. Formation Of Table Salt Equation.
From www.shutterstock.com
Process Dissociation Table Salt Sodium Chloride Stock Vector (Royalty Formation Of Table Salt Equation the molecular formula of table salt—sodium chloride—is nacl. 2na (s) + cl₂ (g) → 2nacl (s). revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. It is an ionic compound which consists of a chloride. this reaction is represented as: sodium and chlorine react. Formation Of Table Salt Equation.
From www.youtube.com
Table Salt from the Elements YouTube Formation Of Table Salt Equation Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. Both methods result in the formation of sodium chloride, a vital. In the solid lattice, each ion is surrounded by six ions. . Formation Of Table Salt Equation.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Formation Of Table Salt Equation Nacl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. this reaction is represented as: 2na(s) + cl 2 (g) → 2nacl(s) physical properties of sodium chloride thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. In the solid lattice, each. Formation Of Table Salt Equation.
From elchoroukhost.net
Chemical Equation For Table Salt Elcho Table Formation Of Table Salt Equation thus sodium chloride, also known as table salt, is formed when the sodium atom loses one electron from its. It is an ionic compound which consists of a chloride. the molecular formula of table salt—sodium chloride—is nacl. In the solid lattice, each ion is surrounded by six ions. 2na(s) + cl 2 (g) → 2nacl(s) physical properties of. Formation Of Table Salt Equation.
From www.slideserve.com
PPT Chapter 3 Matter and Energy PowerPoint Presentation, free Formation Of Table Salt Equation 2na (s) + cl₂ (g) → 2nacl (s). revision notes on preparing soluble salts for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. sodium and chlorine react to form sodium chloride, also known as table salt or common salt, which is familiar to nearly everyone on the planet. Nacl is the molecular. Formation Of Table Salt Equation.