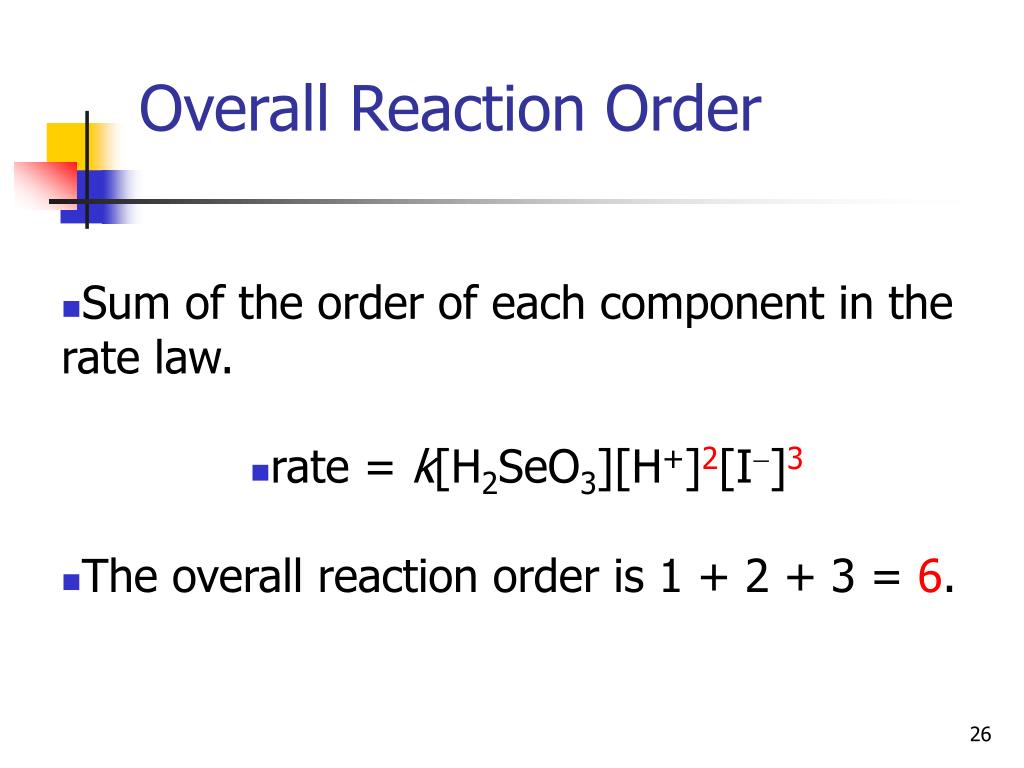
How To Get Overall Reaction . the overall order of the reaction is found by adding up the individual orders. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. our objective is to determine the reaction order by calculating the n from a set of experiments. If m = 1 and n = 1, the overall. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. the overall reaction order is the sum of the orders with respect to each reactant. For example, if the reaction is first order with respect to both a and b (a = 1 and b.
from www.slideserve.com
the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. If m = 1 and n = 1, the overall. the overall reaction order is the sum of the orders with respect to each reactant. the overall order of the reaction is found by adding up the individual orders. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? For example, if the reaction is first order with respect to both a and b (a = 1 and b. our objective is to determine the reaction order by calculating the n from a set of experiments.
PPT Chapter 12 Chemical PowerPoint Presentation, free
How To Get Overall Reaction the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? the overall reaction order is the sum of the orders with respect to each reactant. our objective is to determine the reaction order by calculating the n from a set of experiments. the overall order of the reaction is found by adding up the individual orders. the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. For example, if the reaction is first order with respect to both a and b (a = 1 and b. If m = 1 and n = 1, the overall.
From www.chegg.com
Solved Determine the overall order for a reaction with the How To Get Overall Reaction what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? If m = 1 and n = 1, the overall. For example, if the reaction is first order with respect to both a and b (a = 1 and b. the overall order of the reaction is. How To Get Overall Reaction.
From www.numerade.com
SOLVED Write a balanced overall reaction from these unbalanced half How To Get Overall Reaction our objective is to determine the reaction order by calculating the n from a set of experiments. the overall reaction order is the sum of the orders with respect to each reactant. If m = 1 and n = 1, the overall. the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of. How To Get Overall Reaction.
From www.chegg.com
Solved Overall reaction for formation of suberic acid from How To Get Overall Reaction what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? For example, if the reaction is first order with respect to both a and b (a = 1 and b. the overall order of the reaction is found by adding up the individual orders. the rate. How To Get Overall Reaction.
From www.youtube.com
Reaction Mechanisms and rate laws elementary steps and overall How To Get Overall Reaction each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. the overall reaction order is the sum of the orders with respect to each reactant. our objective is to determine the reaction order by calculating the n from a set of experiments. the overall order of the reaction is found. How To Get Overall Reaction.
From www.chegg.com
Solved What overall reaction consists of the following How To Get Overall Reaction our objective is to determine the reaction order by calculating the n from a set of experiments. For example, if the reaction is first order with respect to both a and b (a = 1 and b. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction?. How To Get Overall Reaction.
From shilohgrohuerta.blogspot.com
Rate of Reaction Calculation How To Get Overall Reaction the overall reaction order is the sum of the orders with respect to each reactant. the overall order of the reaction is found by adding up the individual orders. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. For example, if the reaction is first order with respect to both. How To Get Overall Reaction.
From www.dynamicscience.com.au
redox reactions overall reaction to half reactions exercises solution1 How To Get Overall Reaction what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? our objective is to determine the reaction order by calculating the n from a set of experiments. the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface. How To Get Overall Reaction.
From irenenewssalinas.blogspot.com
How to Calculate Overall Yield of a Multistep Reaction How To Get Overall Reaction the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction?. How To Get Overall Reaction.
From www.chegg.com
Solved Determine the overall reaction order for the How To Get Overall Reaction the overall reaction order is the sum of the orders with respect to each reactant. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. For example, if the reaction. How To Get Overall Reaction.
From www.wikihow.com
3 Ways to Determine Order of Reaction wikiHow How To Get Overall Reaction what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. For example, if the reaction is first order with respect to both a and b (a =. How To Get Overall Reaction.
From www.numerade.com
SOLVED 4. Consider the following reaction scheme [ 2 1 10 Reaction How To Get Overall Reaction For example, if the reaction is first order with respect to both a and b (a = 1 and b. the overall reaction order is the sum of the orders with respect to each reactant. If m = 1 and n = 1, the overall. what is the order of reaction with respect to methanol and ethyl acetate,. How To Get Overall Reaction.
From www.chegg.com
Solved 4a. Consider the reaction below. The overall reaction How To Get Overall Reaction each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. the overall reaction order is the sum of the orders with respect to each reactant. For example, if the reaction is first order with respect to both a and b (a = 1 and b. the overall order of the reaction. How To Get Overall Reaction.
From www.youtube.com
How we get the Correct Balanced Overall Equation of Photosynthesis How To Get Overall Reaction For example, if the reaction is first order with respect to both a and b (a = 1 and b. the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. the. How To Get Overall Reaction.
From slidesharenow.blogspot.com
Overall Order Of Reaction slideshare How To Get Overall Reaction the overall order of the reaction is found by adding up the individual orders. our objective is to determine the reaction order by calculating the n from a set of experiments. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? the rate of a. How To Get Overall Reaction.
From www.slideserve.com
PPT Chapter 12 Chemical PowerPoint Presentation, free How To Get Overall Reaction each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. the overall reaction order is the sum of the orders with respect to each reactant. For example, if the reaction is first order with respect to both a and b (a = 1 and b. If m = 1 and n =. How To Get Overall Reaction.
From brainly.com
what is the overall equation for this chemical reaction? How To Get Overall Reaction For example, if the reaction is first order with respect to both a and b (a = 1 and b. the overall order of the reaction is found by adding up the individual orders. If m = 1 and n = 1, the overall. what is the order of reaction with respect to methanol and ethyl acetate, and. How To Get Overall Reaction.
From www.chegg.com
Solved write a balanced overall reaction from these How To Get Overall Reaction what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? the overall order of the reaction is found by adding up the individual orders. the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. each. How To Get Overall Reaction.
From www.nagwa.com
Question Video Identifying the Overall Equation for the Reaction That How To Get Overall Reaction what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? the overall order of the reaction is found by adding up the individual orders. our objective is to determine the reaction order by calculating the n from a set of experiments. If m = 1 and. How To Get Overall Reaction.
From www.slideserve.com
PPT Overall Photosynthesis Reaction PowerPoint Presentation, free How To Get Overall Reaction our objective is to determine the reaction order by calculating the n from a set of experiments. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. the rate. How To Get Overall Reaction.
From oneclass.com
OneClass Rate law and overall reaction order. I cannt figure out how How To Get Overall Reaction For example, if the reaction is first order with respect to both a and b (a = 1 and b. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. our objective is to determine the reaction order by calculating the n from a set of experiments. what is the order. How To Get Overall Reaction.
From www.slideserve.com
PPT Nonelementary Reaction PowerPoint Presentation, free How To Get Overall Reaction what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? the overall reaction order is the sum of the orders with respect to each reactant. the overall order of the reaction is found by adding up the individual orders. our objective is to determine the. How To Get Overall Reaction.
From simplypsychology.org
házi feladat repertoár Merülnek fel overall rate of reaction How To Get Overall Reaction the overall reaction order is the sum of the orders with respect to each reactant. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. For example, if the reaction is first order with respect to both a and b (a = 1 and b. what is the order of reaction. How To Get Overall Reaction.
From www.chegg.com
Solved What overall reaction consists of the following How To Get Overall Reaction the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. If m = 1 and n = 1, the overall. the overall reaction order is the sum of the orders with. How To Get Overall Reaction.
From study.com
How to Determine the Order of Reaction by Comparing Initial Rates of How To Get Overall Reaction the overall reaction order is the sum of the orders with respect to each reactant. our objective is to determine the reaction order by calculating the n from a set of experiments. the overall order of the reaction is found by adding up the individual orders. If m = 1 and n = 1, the overall. . How To Get Overall Reaction.
From brainly.com
Write the balanced overall reaction for the threestep mechanism. 1 How To Get Overall Reaction each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. the overall order of the reaction is found by adding up the individual orders. For example, if the reaction is first. How To Get Overall Reaction.
From www.homeworklib.com
Give the individual reaction orders for all substances and the overall How To Get Overall Reaction For example, if the reaction is first order with respect to both a and b (a = 1 and b. our objective is to determine the reaction order by calculating the n from a set of experiments. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction?. How To Get Overall Reaction.
From facts.net
11 Fascinating Facts About Reaction Order How To Get Overall Reaction our objective is to determine the reaction order by calculating the n from a set of experiments. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. For example, if the reaction is first order with respect to both a and b (a = 1 and b. the rate of a. How To Get Overall Reaction.
From www.homeworklib.com
How would you combine reactions AC, shown below, to obtain the overall How To Get Overall Reaction For example, if the reaction is first order with respect to both a and b (a = 1 and b. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. . How To Get Overall Reaction.
From www.researchgate.net
Scheme 1 Overall reaction steps Download Scientific Diagram How To Get Overall Reaction If m = 1 and n = 1, the overall. For example, if the reaction is first order with respect to both a and b (a = 1 and b. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. what is the order of reaction with respect to methanol and ethyl. How To Get Overall Reaction.
From www.showme.com
Order of a reaction Science, Chemistry, Rates Of Reaction ShowMe How To Get Overall Reaction our objective is to determine the reaction order by calculating the n from a set of experiments. For example, if the reaction is first order with respect to both a and b (a = 1 and b. If m = 1 and n = 1, the overall. what is the order of reaction with respect to methanol and. How To Get Overall Reaction.
From www.youtube.com
Chemistry Overall Order of Reaction YouTube How To Get Overall Reaction the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. the overall reaction order is the sum of the orders with respect to each reactant. If m = 1 and n = 1, the overall. our objective is to determine the reaction order by calculating the n. How To Get Overall Reaction.
From www.numerade.com
SOLVEDConsider the following reaction energy diagram (a) How many How To Get Overall Reaction the overall order of the reaction is found by adding up the individual orders. If m = 1 and n = 1, the overall. the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants, surface area,. For example, if the reaction is first order with respect to both a and. How To Get Overall Reaction.
From www.tes.com
Energy Changes Endothermic and Exothermic Reactions Edexcel 91 How To Get Overall Reaction If m = 1 and n = 1, the overall. each individual reaction, which is called an elementary reaction, involves one, two, or (rarely) three atoms,. the overall reaction order is the sum of the orders with respect to each reactant. the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity). How To Get Overall Reaction.
From www.doubtnut.com
Determining the overall reaction and the molecularity of an elementary How To Get Overall Reaction If m = 1 and n = 1, the overall. the overall order of the reaction is found by adding up the individual orders. the overall reaction order is the sum of the orders with respect to each reactant. the rate of a chemical reaction is determined—and altered—by many factors, including the nature (of reactivity) of reactants,. How To Get Overall Reaction.
From www.youtube.com
Predict overall reaction and rate law with rate determining step YouTube How To Get Overall Reaction our objective is to determine the reaction order by calculating the n from a set of experiments. If m = 1 and n = 1, the overall. what is the order of reaction with respect to methanol and ethyl acetate, and what is the overall order of reaction? each individual reaction, which is called an elementary reaction,. How To Get Overall Reaction.