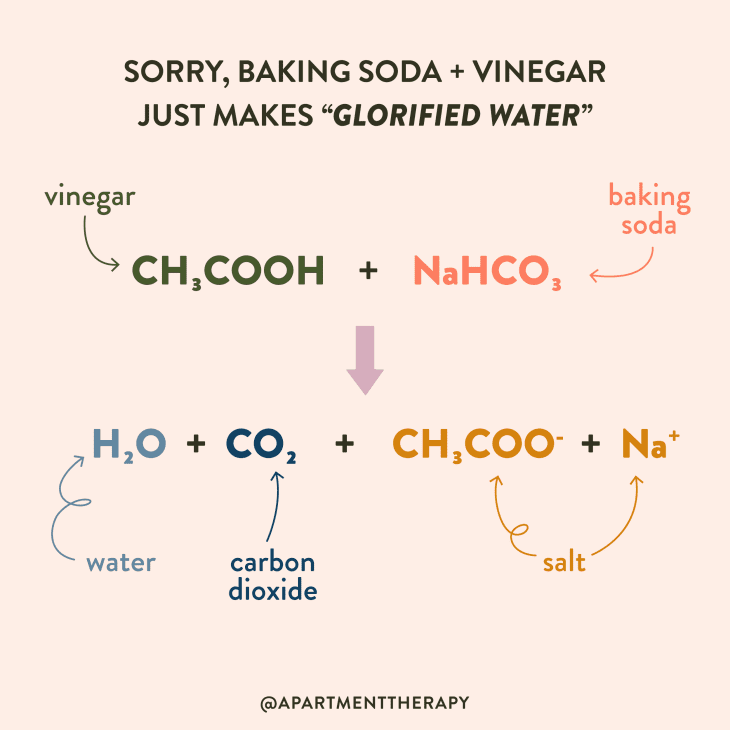What Is The Chemical Equation For Vinegar . Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The chemical equation for vinegar is as follows: The structural formula for acetic acid is ch 3 cooh. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The concentration of the acetic acid is variable. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Vinegar is considered a type of weak. The molecular formula for water is h 2 o.
from www.apartmenttherapy.com
The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The molecular formula for water is h 2 o. The structural formula for acetic acid is ch 3 cooh. The concentration of the acetic acid is variable. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Vinegar is considered a type of weak. The chemical equation for vinegar is as follows:
Don't Mix Baking Soda and Vinegar for Cleaning Apartment Therapy
What Is The Chemical Equation For Vinegar The chemical equation for vinegar is as follows: The structural formula for acetic acid is ch 3 cooh. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The molecular formula for water is h 2 o. Vinegar is considered a type of weak. The concentration of the acetic acid is variable. The chemical equation for vinegar is as follows:
From ar.inspiredpencil.com
Chemical Formula For Vinegar What Is The Chemical Equation For Vinegar Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The structural formula for acetic acid is ch 3 cooh. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. Vinegar is considered a type of weak.. What Is The Chemical Equation For Vinegar.
From studyonline.blog
What Is a Chemical Equation? Definition and Examples What Is The Chemical Equation For Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. The molecular formula for water is h 2 o. Acetic acid, often recognized by its. What Is The Chemical Equation For Vinegar.
From shotprofessional22.gitlab.io
Ideal What Is The Balanced Chemical Equation For Vinegar And Baking What Is The Chemical Equation For Vinegar The chemical equation for vinegar is as follows: The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Vinegar is considered a type of weak.. What Is The Chemical Equation For Vinegar.
From www.coursehero.com
[Solved] 6. The equation for the reaction between baking soda and What Is The Chemical Equation For Vinegar The chemical equation for vinegar is as follows: Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. Vinegar is considered a type of weak. The molecular formula for water is h 2 o. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich. What Is The Chemical Equation For Vinegar.
From ar.inspiredpencil.com
Vinegar Formula What Is The Chemical Equation For Vinegar The chemical equation for vinegar is as follows: Vinegar is considered a type of weak. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The molecular formula for water is h 2 o. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts. What Is The Chemical Equation For Vinegar.
From ar.inspiredpencil.com
Chemical Formula For Vinegar What Is The Chemical Equation For Vinegar The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Vinegar is considered a type of weak. The concentration of the acetic acid is variable. The chemical equation for vinegar is as follows: The molecular formula for water is h 2 o. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid. What Is The Chemical Equation For Vinegar.
From tikalon.com
Tikalon Blog by Dev Gualtieri What Is The Chemical Equation For Vinegar The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# The chemical equation for vinegar is as follows: The structural formula for acetic acid is ch 3 cooh. The molecular formula for water is h 2 o. The concentration of the acetic acid is variable. Vinegar is considered a type of weak. The overall chemical reaction between baking soda (sodium bicarbonate). What Is The Chemical Equation For Vinegar.
From chemistry291.blogspot.com
Baking Soda and Vinegar Chemical Reaction ExplanationNaHCO3 + CH3COOH What Is The Chemical Equation For Vinegar The molecular formula for water is h 2 o. Vinegar is considered a type of weak. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The concentration of the acetic acid is variable. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# The chemical equation. What Is The Chemical Equation For Vinegar.
From www.saubhaya.com
What Is The Chemical Makeup Of Vinegar Saubhaya Makeup What Is The Chemical Equation For Vinegar Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The structural formula for acetic acid is ch 3 cooh. The molecular formula for water is h. What Is The Chemical Equation For Vinegar.
From www.chegg.com
Solved 1. write the balanced equation for the vinegar What Is The Chemical Equation For Vinegar The structural formula for acetic acid is ch 3 cooh. The concentration of the acetic acid is variable. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions,. What Is The Chemical Equation For Vinegar.
From www.shutterstock.com
255 Vinegar formula Images, Stock Photos & Vectors Shutterstock What Is The Chemical Equation For Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Vinegar is considered a type of weak. Vinegar consists of acetic acid (ch 3 cooh),. What Is The Chemical Equation For Vinegar.
From aceticacidvinegar.weebly.com
VINEGAR General Information What Is The Chemical Equation For Vinegar The concentration of the acetic acid is variable. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Acetic acid, often recognized by its more. What Is The Chemical Equation For Vinegar.
From signalticket9.pythonanywhere.com
Marvelous Vinegar Plus Baking Soda Chemical Equation Balanced For And What Is The Chemical Equation For Vinegar The concentration of the acetic acid is variable. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The chemical equation for vinegar is as follows: The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# The molecular formula for water is h 2 o. Vinegar is considered a type of weak.. What Is The Chemical Equation For Vinegar.
From mugeek.vidalondon.net
What Is The Chemical Makeup Of Vinegar Mugeek Vidalondon What Is The Chemical Equation For Vinegar The molecular formula for water is h 2 o. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Acetic acid, often recognized by its. What Is The Chemical Equation For Vinegar.
From www.thoughtco.com
Vinegar (Acetic Acid) Molecular and Structural Formula What Is The Chemical Equation For Vinegar The molecular formula for water is h 2 o. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include. What Is The Chemical Equation For Vinegar.
From shotprofessional22.gitlab.io
Ideal What Is The Balanced Chemical Equation For Vinegar And Baking What Is The Chemical Equation For Vinegar The concentration of the acetic acid is variable. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# The structural formula for acetic acid is ch 3 cooh. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance. What Is The Chemical Equation For Vinegar.
From www.thoughtco.com
Chemical Composition of Vinegar What Is The Chemical Equation For Vinegar Vinegar is considered a type of weak. The concentration of the acetic acid is variable. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The molecular formula for water is h 2 o. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak. What Is The Chemical Equation For Vinegar.
From team-cartwright.com
Fizzy Heart Science Experiment Team Cartwright What Is The Chemical Equation For Vinegar The concentration of the acetic acid is variable. The molecular formula for water is h 2 o. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one. What Is The Chemical Equation For Vinegar.
From ar.inspiredpencil.com
Vinegar Formula What Is The Chemical Equation For Vinegar The concentration of the acetic acid is variable. Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications.. What Is The Chemical Equation For Vinegar.
From www.numerade.com
SOLVED Write a chemical equation to describe the reaction of vinegar What Is The Chemical Equation For Vinegar Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The chemical equation for vinegar is as follows: The molecular formula for water is h 2 o. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Vinegar is considered a type of weak. The structural formula for acetic acid is ch. What Is The Chemical Equation For Vinegar.
From asideload7.gitlab.io
Amazing Balanced Chemical Equation Of Vinegar And Baking Soda What Is The Chemical Equation For Vinegar The molecular formula for water is h 2 o. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The structural formula for acetic acid is ch 3 cooh. The chemical equation for vinegar is as follows: The overall chemical reaction between. What Is The Chemical Equation For Vinegar.
From treatybottle13.pythonanywhere.com
Amazing Word Equation For Baking Soda And Vinegar What Is Magnitude Of What Is The Chemical Equation For Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with. What Is The Chemical Equation For Vinegar.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium What Is The Chemical Equation For Vinegar The concentration of the acetic acid is variable. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The structural formula for acetic acid is ch 3 cooh. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of. What Is The Chemical Equation For Vinegar.
From treatybottle13.pythonanywhere.com
Ace Chemical Equation For Vinegar Ap Reference Sheet What Is The Chemical Equation For Vinegar The chemical equation for vinegar is as follows: The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The concentration of the acetic acid is variable. The molecular formula for water is h 2 o. Vinegar consists. What Is The Chemical Equation For Vinegar.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium What Is The Chemical Equation For Vinegar Vinegar is considered a type of weak. The concentration of the acetic acid is variable. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# The chemical equation for vinegar is as follows: The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic. What Is The Chemical Equation For Vinegar.
From www.compoundchem.com
Compound Interest The sour science of vinegar varieties What Is The Chemical Equation For Vinegar Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Vinegar is considered a type of weak. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The. What Is The Chemical Equation For Vinegar.
From shotprofessional22.gitlab.io
Supreme Baking Soda And Vinegar Word Equation C Formula Physics What Is The Chemical Equation For Vinegar The chemical equation for vinegar is as follows: The concentration of the acetic acid is variable. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate. What Is The Chemical Equation For Vinegar.
From www.coursehero.com
[Solved] 6. The equation for the reaction between baking soda and What Is The Chemical Equation For Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with. What Is The Chemical Equation For Vinegar.
From www.slideserve.com
PPT Vinegar fermentation PowerPoint Presentation, free download ID What Is The Chemical Equation For Vinegar The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# The molecular formula for water is h 2 o. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions,. What Is The Chemical Equation For Vinegar.
From thoughtfullysustainable.com
How to Explain the Chemistry of Cleaning with Vinegar Thoughtfully What Is The Chemical Equation For Vinegar The chemical equation for vinegar is as follows: The molecular formula for water is h 2 o. The concentration of the acetic acid is variable. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of. What Is The Chemical Equation For Vinegar.
From www.youtube.com
Chemical Formula for Vinegar (Acetic Acid or Ethanoic Acid) YouTube What Is The Chemical Equation For Vinegar Vinegar consists of acetic acid (ch 3 cooh), water, and trace amounts of other chemicals, which may include flavorings. The concentration of the acetic acid is variable. The structural formula for acetic acid is ch 3 cooh. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# Acetic acid, often recognized by its more common name, vinegar, is a versatile substance. What Is The Chemical Equation For Vinegar.
From www.apartmenttherapy.com
Don't Mix Baking Soda and Vinegar for Cleaning Apartment Therapy What Is The Chemical Equation For Vinegar The concentration of the acetic acid is variable. The molecular formula for water is h 2 o. Vinegar is considered a type of weak. The structural formula for acetic acid is ch 3 cooh. The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of. What Is The Chemical Equation For Vinegar.
From school.careers360.com
ethanol formula Overview, Structure, Properties & Uses What Is The Chemical Equation For Vinegar The chemical equation for vinegar is as follows: Vinegar is considered a type of weak. The molecular formula for water is h 2 o. The combination of baking soda and vinegar is #nahco_3+ch_3cooh=ch_3coona+h_2o+co_2# The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid. What Is The Chemical Equation For Vinegar.
From www.ingridscience.ca
Baking soda and vinegar ingridscience.ca What Is The Chemical Equation For Vinegar The overall chemical reaction between baking soda (sodium bicarbonate) and vinegar (weak acetic acid) is one mole of solid sodium bicarbonate reacts with one mole of liquid acetic acid to produce one mole each of carbon dioxide gas, liquid water, sodium ions, and acetate ions. The concentration of the acetic acid is variable. Vinegar is considered a type of weak.. What Is The Chemical Equation For Vinegar.
From www.slideserve.com
PPT Baking Soda/Vinegar Stoichiometry Lab PowerPoint Presentation What Is The Chemical Equation For Vinegar The chemical equation for vinegar is as follows: Vinegar is considered a type of weak. The molecular formula for water is h 2 o. Acetic acid, often recognized by its more common name, vinegar, is a versatile substance with a rich history and a wide range of applications. The structural formula for acetic acid is ch 3 cooh. The concentration. What Is The Chemical Equation For Vinegar.