Why Chlorine Electron Affinity . Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Electron affinity of chlorine is 349 kj/mol. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Chlorine gains a stable octet when it captures an electron. This makes the fluoride anion so.
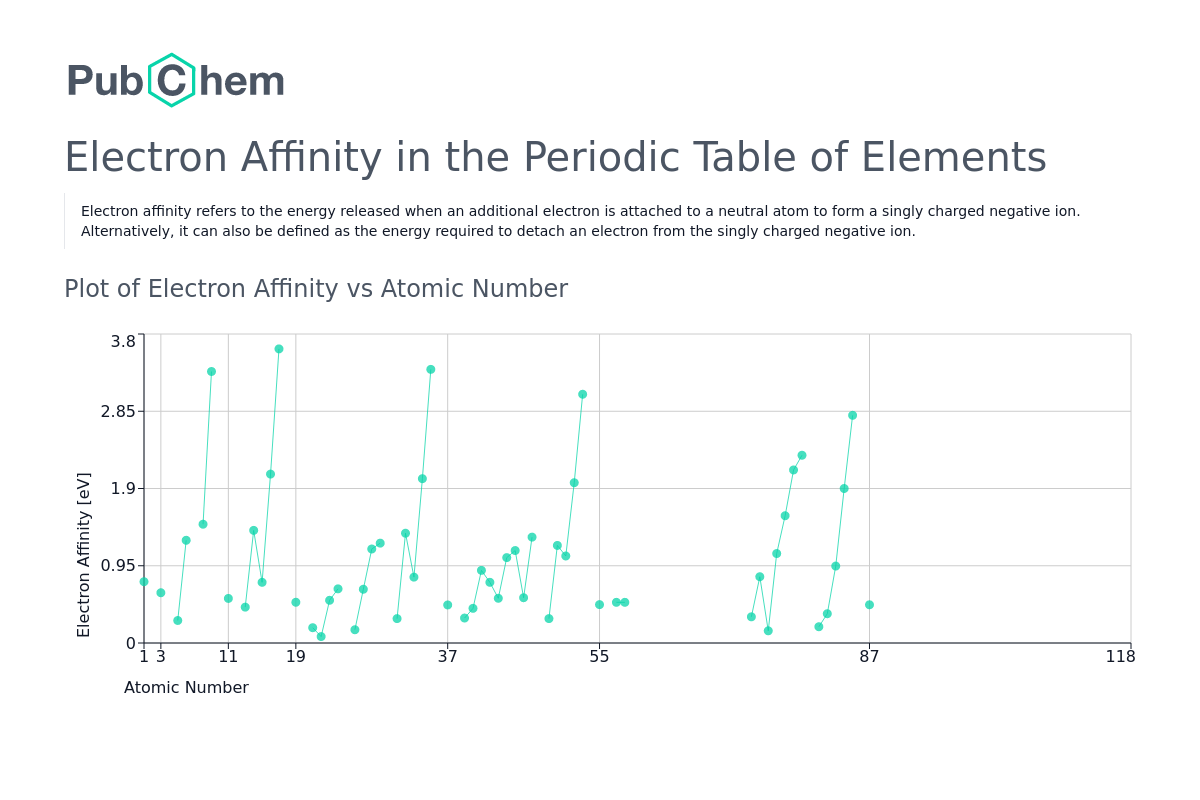
from pubchem.ncbi.nlm.nih.gov
This makes the fluoride anion so. Electron affinity of chlorine is 349 kj/mol. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Chlorine gains a stable octet when it captures an electron. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size.
Electron Affinity Periodic Table of Elements PubChem
Why Chlorine Electron Affinity Chlorine gains a stable octet when it captures an electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of chlorine is 349 kj/mol. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Chlorine gains a stable octet when it captures an electron. This makes the fluoride anion so.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.1.2 Electron Affinity & Trends of Group 16 Why Chlorine Electron Affinity The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. In chemistry and atomic physics, the electron affinity of an atom or. Why Chlorine Electron Affinity.
From socratic.org
Which following pairs of atoms, have a lower electron affinity? a) Ca,K Why Chlorine Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to. Why Chlorine Electron Affinity.
From brainly.in
The electron affinity of chlorine is 3.7 eV1 gram of chlorine is Why Chlorine Electron Affinity Chlorine gains a stable octet when it captures an electron. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an. Why Chlorine Electron Affinity.
From www.youtube.com
Why Electron Affinity of Fluorine is lower than Chlorine by Tariq Why Chlorine Electron Affinity Electron affinity of chlorine is 349 kj/mol. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Chlorine gains a stable octet when it captures an electron. The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Electron affinity is defined as the change in energy (in kj/mole) of a. Why Chlorine Electron Affinity.
From www.meritnation.com
Does chlorine have higher electron affinity value than fluorine Why Chlorine Electron Affinity Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Electron affinity. Why Chlorine Electron Affinity.
From www.studypool.com
SOLUTION why electron affinity of flourine is less than chlorine Why Chlorine Electron Affinity The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. This makes the. Why Chlorine Electron Affinity.
From slideplayer.com
Learning Objectives General trends of group 17 elements ppt download Why Chlorine Electron Affinity Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. The element with the highest electron affinity is. Why Chlorine Electron Affinity.
From www.youtube.com
Periodic Trends Electron Affinity. YouTube Why Chlorine Electron Affinity Electron affinity of chlorine is 349 kj/mol. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Chlorine gains a stable octet when it captures an electron. The. Why Chlorine Electron Affinity.
From www.chemistrylearner.com
Electron Affinity Definition, Chart & Trend in Periodic Table Why Chlorine Electron Affinity Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Chlorine gains a stable octet when it captures an electron. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. The chlorine atom has the most negative electron affinity of any. Why Chlorine Electron Affinity.
From www.nuclear-power.com
Fluorine Electron Affinity Electronegativity Ionization Energy of Why Chlorine Electron Affinity Chlorine gains a stable octet when it captures an electron. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the. Why Chlorine Electron Affinity.
From pubchem.ncbi.nlm.nih.gov
Electron Affinity Periodic Table of Elements PubChem Why Chlorine Electron Affinity The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Since chlorine's outermost orbital is a 3p orbital, there is more space,. Why Chlorine Electron Affinity.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Why Chlorine Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. This makes the fluoride anion so. The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Electron affinity is defined as the change in energy (in. Why Chlorine Electron Affinity.
From chemistry.stackexchange.com
periodic trends If fluorine has a lower electron affinity than Why Chlorine Electron Affinity This makes the fluoride anion so. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. The chlorine. Why Chlorine Electron Affinity.
From www.toppr.com
The electron affinity (1) Of carbon is greater than oxygen (2) Of Why Chlorine Electron Affinity This makes the fluoride anion so. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The chlorine atom has the most negative electron affinity of any element, which means that more. Why Chlorine Electron Affinity.
From brainly.in
Why chlorine has a higher electron affinity than fluorine? Brainly.in Why Chlorine Electron Affinity This makes the fluoride anion so. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. The chlorine atom has the most negative electron affinity of any. Why Chlorine Electron Affinity.
From www.sliderbase.com
Periodic Behavior Presentation Chemistry Why Chlorine Electron Affinity Electron affinity of chlorine is 349 kj/mol. Chlorine gains a stable octet when it captures an electron. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are. Why Chlorine Electron Affinity.
From mavink.com
Periodic Table With Electron Affinity Why Chlorine Electron Affinity Electron affinity of chlorine is 349 kj/mol. Chlorine gains a stable octet when it captures an electron. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. In chemistry and atomic physics, the electron affinity of an atom or molecule is. The element with the highest electron affinity is chlorine, with a value of 349. Why Chlorine Electron Affinity.
From valenceelectrons.com
How Many Valence Electrons Does PCl3 Have? Why Chlorine Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Chlorine gains a stable octet when it captures. Why Chlorine Electron Affinity.
From gbu-taganskij.ru
Electron Affinity Definition, Chart Trend In Periodic, 47 OFF Why Chlorine Electron Affinity Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Electron affinity of chlorine is 349 kj/mol. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. This makes the fluoride anion so. Since chlorine's outermost orbital is a 3p orbital,. Why Chlorine Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Why Chlorine Electron Affinity Chlorine gains a stable octet when it captures an electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. The chlorine atom has the most negative electron affinity of any element,. Why Chlorine Electron Affinity.
From www.youtube.com
Cl এর ইলেকট্রন আসক্তির মান F অপেক্ষা বেশি কেন ? Why electron Why Chlorine Electron Affinity Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Chlorine gains a stable octet when it captures an electron. Electron affinity of chlorine is 349 kj/mol. Fluorine,. Why Chlorine Electron Affinity.
From www.meritnation.com
Does chlorine have higher electron affinity value than fluorine Why Chlorine Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Chlorine gains a stable octet when it captures an electron. Since chlorine's outermost orbital is a 3p orbital, there is more space,. Why Chlorine Electron Affinity.
From wisc.pb.unizin.org
D4.4 Periodic Variation in Electron Affinities Chem 109 Fall 2023 Why Chlorine Electron Affinity The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of chlorine is 349 kj/mol. This makes the fluoride anion so. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous. Why Chlorine Electron Affinity.
From ecurrencythailand.com
What Is The Electron Affinity Of Bromine? All Answers Why Chlorine Electron Affinity Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Electron affinity of chlorine is 349 kj/mol. This makes the fluoride anion so. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this. Why Chlorine Electron Affinity.
From eduinput.com
Electron Affinity, definition, examples, significance, factors Why Chlorine Electron Affinity In chemistry and atomic physics, the electron affinity of an atom or molecule is. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. The chlorine atom has the most negative electron affinity of any element, which means that more energy is. Why Chlorine Electron Affinity.
From www.toppr.com
The electron affinity of chlorine is 3.7 eV. 1 gram of chlorine is Why Chlorine Electron Affinity Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Chlorine gains a stable octet when it captures an electron. Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Since chlorine's outermost orbital is a 3p orbital, there is more. Why Chlorine Electron Affinity.
From chemisfast.blogspot.com
Electron affinity and periodic variation of electron affinity. PG Why Chlorine Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. Electron affinity of chlorine is 349 kj/mol. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Fluorine,. Why Chlorine Electron Affinity.
From www.youtube.com
Electron Affinity YouTube Why Chlorine Electron Affinity The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Electron affinity of chlorine is 349 kj/mol. This makes the fluoride anion so. In chemistry and atomic physics, the electron affinity of an atom or molecule is.. Why Chlorine Electron Affinity.
From www.pinterest.co.kr
Electron Affinity Trend and Definition Electron affinity, Ionization Why Chlorine Electron Affinity Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. Electron affinity of chlorine is 349 kj/mol. Chlorine gains a stable octet when it captures an electron. The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine. Why Chlorine Electron Affinity.
From www.youtube.com
Chlorine has ___________ electron affinity than fluorine. YouTube Why Chlorine Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. This makes the fluoride anion so. Electron affinity of chlorine is 349 kj/mol. Chlorine gains a stable octet when it captures an electron. The element with the highest electron affinity is chlorine, with a value of. Why Chlorine Electron Affinity.
From www.youtube.com
Why is electron affinity of chlorine more than thatof fluorine? CLASS Why Chlorine Electron Affinity Electron affinity is defined as the change in energy (in kj/mole) of a neutral atom (in the gaseous phase) when an electron is added. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron affinity of chlorine is 349 kj/mol. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons. Why Chlorine Electron Affinity.
From www.youtube.com
Halogens second electron Affinity is zero Why?Fluorine chlorine bromine Why Chlorine Electron Affinity Electron affinity of chlorine is 349 kj/mol. This makes the fluoride anion so. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Fluorine, though higher than chlorine in the periodic table, has a very small atomic size. In chemistry and atomic. Why Chlorine Electron Affinity.
From 88guru.com
Electron Affinity Definition and Trends in Periodic Table Why Chlorine Electron Affinity Electron affinity of chlorine is 349 kj/mol. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra electron. Chlorine gains a stable octet when it captures an electron. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Electron. Why Chlorine Electron Affinity.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Why Chlorine Electron Affinity The chlorine atom has the most negative electron affinity of any element, which means that more energy is released when an electron is added to a gaseous chlorine atom than to an atom. Since chlorine's outermost orbital is a 3p orbital, there is more space, and the electrons in this orbital are inclined to share this space with an extra. Why Chlorine Electron Affinity.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Why Chlorine Electron Affinity This makes the fluoride anion so. The element with the highest electron affinity is chlorine, with a value of 349 kj/mole. Electron affinity of chlorine is 349 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is. Chlorine gains a stable octet when it captures an electron. Since chlorine's outermost orbital is a 3p orbital,. Why Chlorine Electron Affinity.