Bromine Has More Electron Affinity Than Iodine . As you know, both chlorine and bromine are located in. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Also, bromine has two isotopes: electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. So, why does that happen? chlorine does have a higher electron affinity than bromine. similarly bromine is a more powerful oxidising agent than iodine. without considering the substance too seriously,. 79 br and 81 br. bromine is more reactive than iodine, but not as reactive as chlorine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Fluorine, chlorine, bromine and iodine. Bromine can remove electrons from iodide ions to give.
from www.numerade.com
As you know, both chlorine and bromine are located in. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Also, bromine has two isotopes: without considering the substance too seriously,. bromine is more reactive than iodine, but not as reactive as chlorine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. 79 br and 81 br. So, why does that happen? chlorine does have a higher electron affinity than bromine. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion.
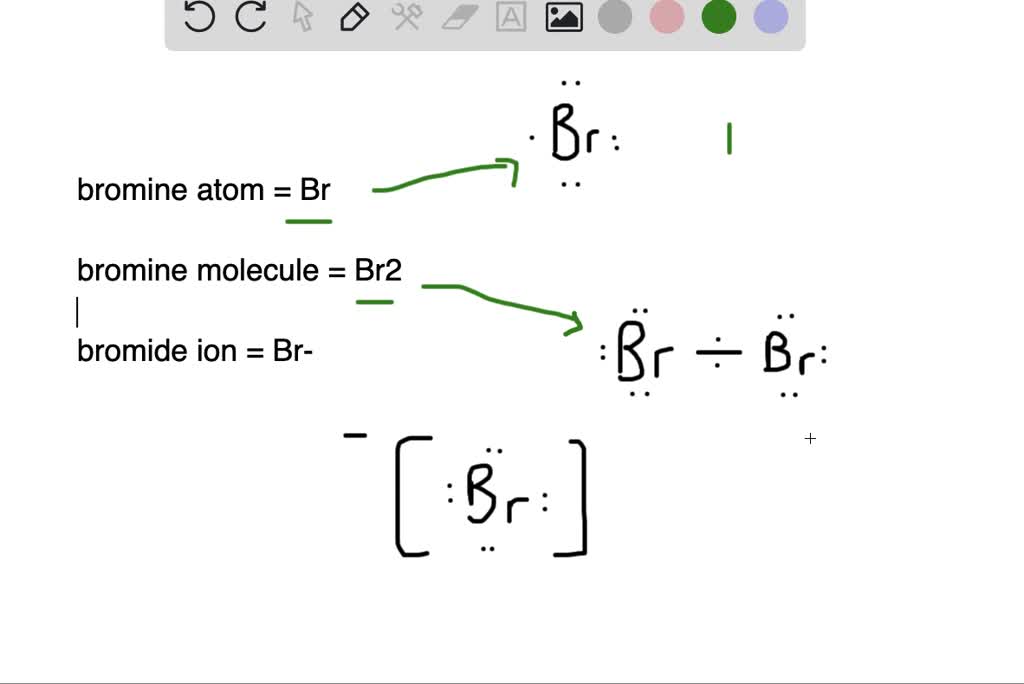
What is the difference between (a) a bromine atom, (b) a bromine
Bromine Has More Electron Affinity Than Iodine Bromine can remove electrons from iodide ions to give. So, why does that happen? similarly bromine is a more powerful oxidising agent than iodine. As you know, both chlorine and bromine are located in. without considering the substance too seriously,. chlorine does have a higher electron affinity than bromine. Bromine can remove electrons from iodide ions to give. bromine is more reactive than iodine, but not as reactive as chlorine. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. 79 br and 81 br. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Fluorine, chlorine, bromine and iodine. Also, bromine has two isotopes:
From www.breakingatom.com
Electron Affinity of The Elements Bromine Has More Electron Affinity Than Iodine similarly bromine is a more powerful oxidising agent than iodine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. chlorine does have a higher electron affinity than bromine. As you know, both chlorine and bromine are located in. without considering the. Bromine Has More Electron Affinity Than Iodine.
From giomwhfig.blob.core.windows.net
Bromine Equation Electron at Alexis Barnhart blog Bromine Has More Electron Affinity Than Iodine similarly bromine is a more powerful oxidising agent than iodine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. without considering the substance too seriously,. Bromine can remove electrons from iodide ions to give. Fluorine, chlorine, bromine and iodine. electron affinity. Bromine Has More Electron Affinity Than Iodine.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has More Electron Affinity Than Iodine So, why does that happen? bromine is more reactive than iodine, but not as reactive as chlorine. 79 br and 81 br. without considering the substance too seriously,. Also, bromine has two isotopes: this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): As you know, both chlorine and. Bromine Has More Electron Affinity Than Iodine.
From www.sciencephoto.com
Bromine and Iodine Stock Image C002/8098 Science Photo Library Bromine Has More Electron Affinity Than Iodine without considering the substance too seriously,. similarly bromine is a more powerful oxidising agent than iodine. Also, bromine has two isotopes: Bromine can remove electrons from iodide ions to give. 79 br and 81 br. chlorine does have a higher electron affinity than bromine. As you know, both chlorine and bromine are located in. 119 rows. Bromine Has More Electron Affinity Than Iodine.
From www.differencebetween.com
Difference Between Bromine and Iodine Compare the Difference Between Bromine Has More Electron Affinity Than Iodine 79 br and 81 br. So, why does that happen? Bromine can remove electrons from iodide ions to give. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): without considering the substance too seriously,. electron affinity is defined as the energy released when an electron is added to. Bromine Has More Electron Affinity Than Iodine.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Has More Electron Affinity Than Iodine electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): similarly bromine is a more powerful oxidising agent than iodine. 79 br and 81 br. without considering the substance. Bromine Has More Electron Affinity Than Iodine.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Bromine Has More Electron Affinity Than Iodine chlorine does have a higher electron affinity than bromine. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): bromine is more reactive than iodine, but not as reactive as chlorine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is. Bromine Has More Electron Affinity Than Iodine.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Bromine Has More Electron Affinity Than Iodine 79 br and 81 br. Fluorine, chlorine, bromine and iodine. chlorine does have a higher electron affinity than bromine. Bromine can remove electrons from iodide ions to give. So, why does that happen? electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. As you know, both chlorine and. Bromine Has More Electron Affinity Than Iodine.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Bromine Has More Electron Affinity Than Iodine without considering the substance too seriously,. 79 br and 81 br. Also, bromine has two isotopes: this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): similarly bromine is a more powerful oxidising agent than iodine. As you know, both chlorine and bromine are located in. electron affinity. Bromine Has More Electron Affinity Than Iodine.
From ecurrencythailand.com
Which Of The Following Has Highest Electron Affinity Of Fluorine Bromine Has More Electron Affinity Than Iodine without considering the substance too seriously,. bromine is more reactive than iodine, but not as reactive as chlorine. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): similarly bromine is a more powerful oxidising agent than iodine. Also, bromine has two isotopes: Bromine can remove electrons from. Bromine Has More Electron Affinity Than Iodine.
From www.nuclear-power.com
Electron Affinity Bromine Has More Electron Affinity Than Iodine So, why does that happen? electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. Fluorine, chlorine, bromine and iodine. without considering the substance too seriously,. 79 br and 81 br. similarly bromine is a more powerful oxidising agent than iodine. chlorine does have a higher electron. Bromine Has More Electron Affinity Than Iodine.
From www.doubtnut.com
The electron affinity of bromine atom is equal to the..........of brom Bromine Has More Electron Affinity Than Iodine So, why does that happen? 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. chlorine does have a higher electron affinity than bromine. without considering the substance too seriously,. Also, bromine has two isotopes: Fluorine, chlorine, bromine and iodine. similarly bromine. Bromine Has More Electron Affinity Than Iodine.
From www.gauthmath.com
Why is the atomic radius of iodine larger than the atomic radius of Bromine Has More Electron Affinity Than Iodine Also, bromine has two isotopes: similarly bromine is a more powerful oxidising agent than iodine. Bromine can remove electrons from iodide ions to give. As you know, both chlorine and bromine are located in. So, why does that happen? electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion.. Bromine Has More Electron Affinity Than Iodine.
From giomwhfig.blob.core.windows.net
Bromine Equation Electron at Alexis Barnhart blog Bromine Has More Electron Affinity Than Iodine So, why does that happen? similarly bromine is a more powerful oxidising agent than iodine. Fluorine, chlorine, bromine and iodine. chlorine does have a higher electron affinity than bromine. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): As you know, both chlorine and bromine are located in.. Bromine Has More Electron Affinity Than Iodine.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Has More Electron Affinity Than Iodine electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. Bromine can remove electrons from iodide ions to give. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): similarly bromine is a more powerful oxidising agent than iodine. So,. Bromine Has More Electron Affinity Than Iodine.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Bromine Has More Electron Affinity Than Iodine Fluorine, chlorine, bromine and iodine. As you know, both chlorine and bromine are located in. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. 79 br and 81 br. similarly bromine is a more powerful oxidising agent than iodine. this page discusses. Bromine Has More Electron Affinity Than Iodine.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Bromine Has More Electron Affinity Than Iodine this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): As you know, both chlorine and bromine are located in. Fluorine, chlorine, bromine and iodine. without considering the substance too seriously,. chlorine does have a higher electron affinity than bromine. bromine is more reactive than iodine, but not. Bromine Has More Electron Affinity Than Iodine.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of Bromine Has More Electron Affinity Than Iodine this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): similarly bromine is a more powerful oxidising agent than iodine. Fluorine, chlorine, bromine and iodine. without considering the substance too seriously,. Bromine can remove electrons from iodide ions to give. 79 br and 81 br. bromine is more. Bromine Has More Electron Affinity Than Iodine.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Has More Electron Affinity Than Iodine So, why does that happen? Bromine can remove electrons from iodide ions to give. bromine is more reactive than iodine, but not as reactive as chlorine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. chlorine does have a higher electron affinity. Bromine Has More Electron Affinity Than Iodine.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has More Electron Affinity Than Iodine 79 br and 81 br. Also, bromine has two isotopes: without considering the substance too seriously,. bromine is more reactive than iodine, but not as reactive as chlorine. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. this page discusses the trends in the atomic and. Bromine Has More Electron Affinity Than Iodine.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Bromine Has More Electron Affinity Than Iodine chlorine does have a higher electron affinity than bromine. similarly bromine is a more powerful oxidising agent than iodine. As you know, both chlorine and bromine are located in. without considering the substance too seriously,. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost. Bromine Has More Electron Affinity Than Iodine.
From www.pinterest.com
Electron Affinity Electron affinity, Periodic table, Chemistry for kids Bromine Has More Electron Affinity Than Iodine So, why does that happen? As you know, both chlorine and bromine are located in. Fluorine, chlorine, bromine and iodine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. without considering the substance too seriously,. electron affinity is defined as the energy. Bromine Has More Electron Affinity Than Iodine.
From pediabay.com
Electron Affinity Chart of Elements (With Periodic Table) Pediabay Bromine Has More Electron Affinity Than Iodine chlorine does have a higher electron affinity than bromine. Also, bromine has two isotopes: without considering the substance too seriously,. As you know, both chlorine and bromine are located in. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): electron affinity is defined as the energy released. Bromine Has More Electron Affinity Than Iodine.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) Bromine Has More Electron Affinity Than Iodine 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Bromine can remove electrons from iodide ions to give. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Also, bromine has two isotopes: As you. Bromine Has More Electron Affinity Than Iodine.
From giomwhfig.blob.core.windows.net
Bromine Equation Electron at Alexis Barnhart blog Bromine Has More Electron Affinity Than Iodine without considering the substance too seriously,. Bromine can remove electrons from iodide ions to give. Also, bromine has two isotopes: similarly bromine is a more powerful oxidising agent than iodine. bromine is more reactive than iodine, but not as reactive as chlorine. Fluorine, chlorine, bromine and iodine. 119 rows electron affinity is the amount of energy. Bromine Has More Electron Affinity Than Iodine.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has More Electron Affinity Than Iodine chlorine does have a higher electron affinity than bromine. So, why does that happen? 79 br and 81 br. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Also, bromine has two isotopes: Bromine can remove electrons from iodide ions to give. As. Bromine Has More Electron Affinity Than Iodine.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine Has More Electron Affinity Than Iodine chlorine does have a higher electron affinity than bromine. without considering the substance too seriously,. 79 br and 81 br. similarly bromine is a more powerful oxidising agent than iodine. So, why does that happen? As you know, both chlorine and bromine are located in. bromine is more reactive than iodine, but not as reactive as. Bromine Has More Electron Affinity Than Iodine.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Has More Electron Affinity Than Iodine 79 br and 81 br. Bromine can remove electrons from iodide ions to give. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. chlorine does have a higher electron affinity than bromine. Also, bromine has two isotopes: bromine is more reactive than. Bromine Has More Electron Affinity Than Iodine.
From exorwrmka.blob.core.windows.net
Bromine Of Electron Affinity at Curtis Phillips blog Bromine Has More Electron Affinity Than Iodine As you know, both chlorine and bromine are located in. similarly bromine is a more powerful oxidising agent than iodine. electron affinity is defined as the energy released when an electron is added to a gaseous atom or ion. Fluorine, chlorine, bromine and iodine. without considering the substance too seriously,. bromine is more reactive than iodine,. Bromine Has More Electron Affinity Than Iodine.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Bromine Has More Electron Affinity Than Iodine So, why does that happen? this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): 79 br and 81 br. Bromine can remove electrons from iodide ions to give. Also, bromine has two isotopes: similarly bromine is a more powerful oxidising agent than iodine. chlorine does have a higher. Bromine Has More Electron Affinity Than Iodine.
From lambdageeks.com
Bromine Electron Configuration 7 Easy Steps on How to Write Bromine Has More Electron Affinity Than Iodine chlorine does have a higher electron affinity than bromine. Bromine can remove electrons from iodide ions to give. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. electron affinity is defined as the energy released when an electron is added to a. Bromine Has More Electron Affinity Than Iodine.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Has More Electron Affinity Than Iodine 79 br and 81 br. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. Bromine can remove electrons from iodide ions to give. electron. Bromine Has More Electron Affinity Than Iodine.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Bromine Has More Electron Affinity Than Iodine Bromine can remove electrons from iodide ions to give. Fluorine, chlorine, bromine and iodine. 119 rows electron affinity is the amount of energy change (δe) that occurs when an electron is added in the outermost shell of an. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Also, bromine. Bromine Has More Electron Affinity Than Iodine.
From www.schoolmykids.com
Compare Bromine vs Iodine Periodic Table Element Comparison Compare Bromine Has More Electron Affinity Than Iodine chlorine does have a higher electron affinity than bromine. 79 br and 81 br. So, why does that happen? Fluorine, chlorine, bromine and iodine. bromine is more reactive than iodine, but not as reactive as chlorine. this page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Also, bromine has. Bromine Has More Electron Affinity Than Iodine.
From mavink.com
Periodic Table With Electron Affinity Bromine Has More Electron Affinity Than Iodine So, why does that happen? chlorine does have a higher electron affinity than bromine. bromine is more reactive than iodine, but not as reactive as chlorine. Also, bromine has two isotopes: As you know, both chlorine and bromine are located in. 79 br and 81 br. this page discusses the trends in the atomic and physical properties. Bromine Has More Electron Affinity Than Iodine.