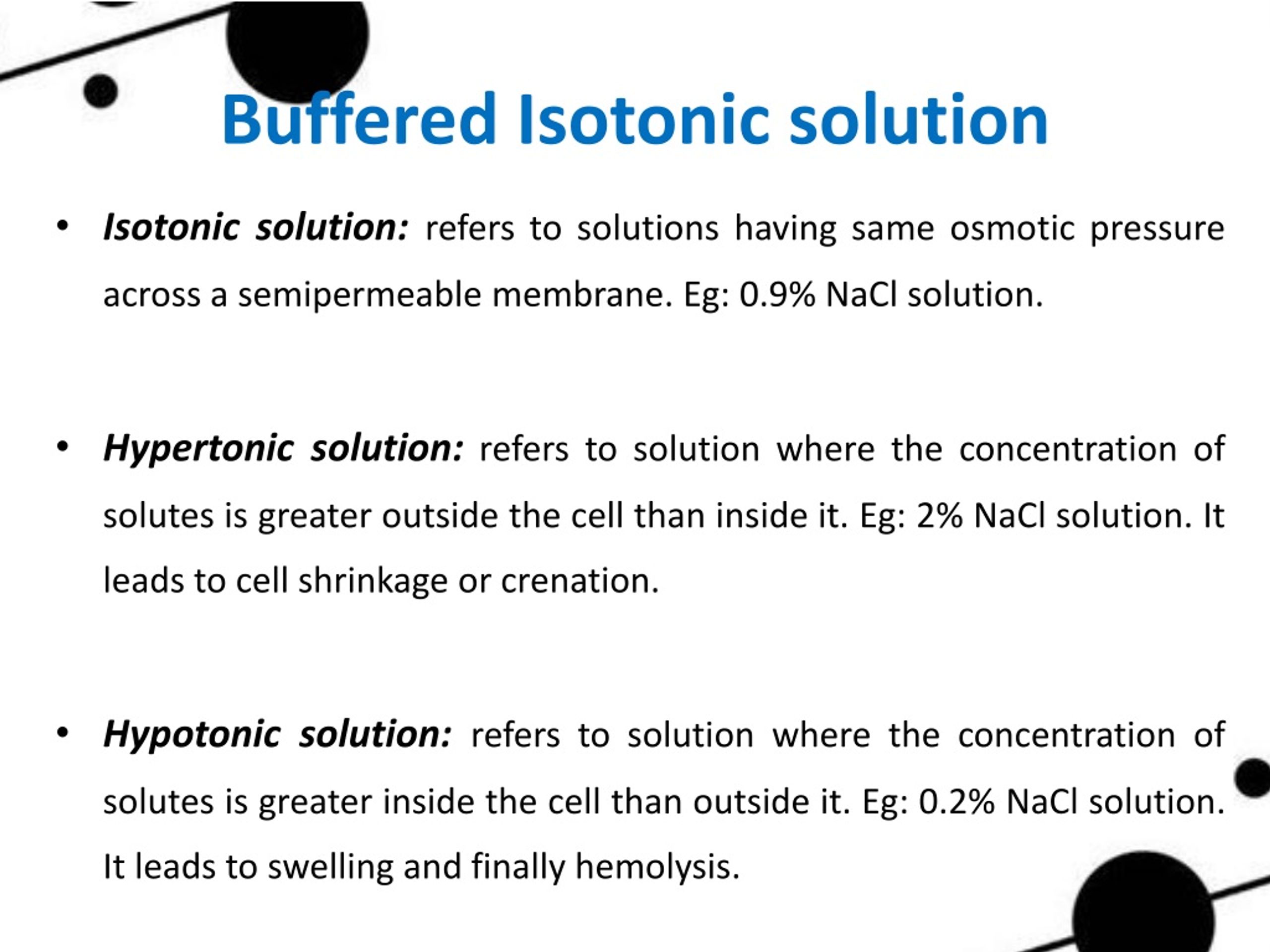
Explain Buffer Solution In Biology . They have maximal buffering capacity at a ph = pka. ph, acids, bases and buffers | introduction to biology. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. to review, buffer solutions contain a weak acid and its conjugate base. By the end of this section, you will be able to: the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. In this way, a biological. Because these acids/bases are “weak,”. buffers are an aqueous solutions of weak acids or bases that minimize a ph change.
from www.slideserve.com
the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. to review, buffer solutions contain a weak acid and its conjugate base. By the end of this section, you will be able to: a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Because these acids/bases are “weak,”. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. ph, acids, bases and buffers | introduction to biology. In this way, a biological. They have maximal buffering capacity at a ph = pka.
PPT UNIT V pH, BUFFERS AND ISOTONIC SOLUTION PowerPoint Presentation
Explain Buffer Solution In Biology They have maximal buffering capacity at a ph = pka. In this way, a biological. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. Because these acids/bases are “weak,”. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. to review, buffer solutions contain a weak acid and its conjugate base. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. They have maximal buffering capacity at a ph = pka. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. By the end of this section, you will be able to: ph, acids, bases and buffers | introduction to biology.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Explain Buffer Solution In Biology the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. to review, buffer solutions contain a weak acid and its conjugate base. Because these acids/bases are “weak,”. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. ph, acids,. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Explain Buffer Solution In Biology the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. They have maximal buffering capacity at a ph = pka. ph, acids, bases and buffers | introduction to biology. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way,. Explain Buffer Solution In Biology.
From studylib.net
Buffer Solutions Explain Buffer Solution In Biology to review, buffer solutions contain a weak acid and its conjugate base. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. ph, acids, bases and buffers | introduction to biology. a biological. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Explain Buffer Solution In Biology to review, buffer solutions contain a weak acid and its conjugate base. Because these acids/bases are “weak,”. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. By the end of this section, you will be able to: In this way, a biological. a biological buffer is an organic substance that has. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Explain Buffer Solution In Biology In this way, a biological. They have maximal buffering capacity at a ph = pka. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. By the end of this section, you will. Explain Buffer Solution In Biology.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Explain Buffer Solution In Biology They have maximal buffering capacity at a ph = pka. ph, acids, bases and buffers | introduction to biology. By the end of this section, you will be able to: the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. Because these acids/bases are “weak,”. the buffer. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Explain Buffer Solution In Biology buffers are an aqueous solutions of weak acids or bases that minimize a ph change. to review, buffer solutions contain a weak acid and its conjugate base. They have maximal buffering capacity at a ph = pka. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. By the end. Explain Buffer Solution In Biology.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs Explain Buffer Solution In Biology By the end of this section, you will be able to: to review, buffer solutions contain a weak acid and its conjugate base. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. a biological buffer is an organic substance that has a neutralizing effect on hydrogen. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT Hydrolysis of Salts and pH of Buffer Solutions PowerPoint Explain Buffer Solution In Biology Because these acids/bases are “weak,”. They have maximal buffering capacity at a ph = pka. to review, buffer solutions contain a weak acid and its conjugate base. In this way, a biological. By the end of this section, you will be able to: the purpose of a buffer in a biological system is to maintain intracellular and extracellular. Explain Buffer Solution In Biology.
From exyfnmepw.blob.core.windows.net
Common Molecular Biology Buffers at Donna Davis blog Explain Buffer Solution In Biology They have maximal buffering capacity at a ph = pka. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. buffers are an aqueous solutions of weak acids or bases that minimize. Explain Buffer Solution In Biology.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download Explain Buffer Solution In Biology Because these acids/bases are “weak,”. to review, buffer solutions contain a weak acid and its conjugate base. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. the purpose of a buffer in a. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT BUFFER SOLUTIONS A guide for A level students PowerPoint Explain Buffer Solution In Biology They have maximal buffering capacity at a ph = pka. By the end of this section, you will be able to: In this way, a biological. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation ID2948821 Explain Buffer Solution In Biology ph, acids, bases and buffers | introduction to biology. They have maximal buffering capacity at a ph = pka. In this way, a biological. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Because these acids/bases are “weak,”. a biological buffer is an organic substance that has a neutralizing. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Explain Buffer Solution In Biology They have maximal buffering capacity at a ph = pka. In this way, a biological. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. By the end of this section, you will be able to: a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions.. Explain Buffer Solution In Biology.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Explain Buffer Solution In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this way, a biological. They have maximal buffering capacity at a ph = pka. By the end of this section, you will be able to: Because these acids/bases are “weak,”. to review, buffer solutions contain a weak acid and its. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Explain Buffer Solution In Biology a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. They have maximal buffering capacity at a ph = pka. In this way, a biological. Because these acids/bases are “weak,”. By the end of this section, you will be able to: buffers are an aqueous solutions of weak acids or bases that minimize. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT THEME Acidbase equilibrium in biological systems. Buffer Explain Buffer Solution In Biology the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. They have maximal buffering capacity at a ph = pka. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Explain Buffer Solution In Biology.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses Explain Buffer Solution In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. By the end of this section, you will be able to: buffers are an aqueous solutions of weak acids or bases that minimize a ph change. to review, buffer solutions contain a weak acid and its conjugate base. a. Explain Buffer Solution In Biology.
From www.slideshare.net
2012 topic 18 2 buffer solutions Explain Buffer Solution In Biology Because these acids/bases are “weak,”. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. to review, buffer solutions contain a weak acid and its conjugate base. In this way, a biological. They have maximal buffering capacity at a ph = pka. the purpose of a buffer in a biological. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT Hydrolysis of Salts and pH of Buffer Solutions PowerPoint Explain Buffer Solution In Biology ph, acids, bases and buffers | introduction to biology. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way, a biological. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. They have maximal buffering capacity at a ph = pka. By the end. Explain Buffer Solution In Biology.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution Explain Buffer Solution In Biology Because these acids/bases are “weak,”. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. buffers are an aqueous solutions of weak acids or bases that minimize a ph change.. Explain Buffer Solution In Biology.
From fr.slideshare.net
Buffer solution Explain Buffer Solution In Biology ph, acids, bases and buffers | introduction to biology. They have maximal buffering capacity at a ph = pka. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. Because these acids/bases are “weak,”. By the end of this section, you will be able to: In this way, a biological. the buffer. Explain Buffer Solution In Biology.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination for Biologists Explain Buffer Solution In Biology to review, buffer solutions contain a weak acid and its conjugate base. Because these acids/bases are “weak,”. In this way, a biological. By the end of this section, you will be able to: the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers are an aqueous solutions of weak. Explain Buffer Solution In Biology.
From exyfnmepw.blob.core.windows.net
Common Molecular Biology Buffers at Donna Davis blog Explain Buffer Solution In Biology Because these acids/bases are “weak,”. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Explain Buffer Solution In Biology.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Explain Buffer Solution In Biology buffers are an aqueous solutions of weak acids or bases that minimize a ph change. to review, buffer solutions contain a weak acid and its conjugate base. They have maximal buffering capacity at a ph = pka. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. ph, acids,. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Explain Buffer Solution In Biology a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Because these acids/bases are “weak,”. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. In this way, a biological.. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Explain Buffer Solution In Biology the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Because these acids/bases are “weak,”. By the end of this section, you will be able to: ph, acids, bases and buffers |. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Explain Buffer Solution In Biology buffers are an aqueous solutions of weak acids or bases that minimize a ph change. to review, buffer solutions contain a weak acid and its conjugate base. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way, a biological. They have maximal buffering capacity at a ph = pka.. Explain Buffer Solution In Biology.
From exyfnmepw.blob.core.windows.net
Common Molecular Biology Buffers at Donna Davis blog Explain Buffer Solution In Biology In this way, a biological. By the end of this section, you will be able to: Because these acids/bases are “weak,”. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. ph, acids, bases and buffers | introduction to biology. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT UNIT V pH, BUFFERS AND ISOTONIC SOLUTION PowerPoint Presentation Explain Buffer Solution In Biology Because these acids/bases are “weak,”. By the end of this section, you will be able to: to review, buffer solutions contain a weak acid and its conjugate base. ph, acids, bases and buffers | introduction to biology. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow.. Explain Buffer Solution In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Explain Buffer Solution In Biology the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. In this way, a biological. to review, buffer solutions contain a weak acid and its conjugate base. By the end of this section, you will be able to: Because these acids/bases are “weak,”. buffers are an aqueous solutions of weak. Explain Buffer Solution In Biology.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid Explain Buffer Solution In Biology the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. ph, acids, bases and buffers | introduction to biology. buffers are an aqueous solutions of weak acids or bases that minimize a ph change. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Explain Buffer Solution In Biology.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation Explain Buffer Solution In Biology buffers are an aqueous solutions of weak acids or bases that minimize a ph change. They have maximal buffering capacity at a ph = pka. Because these acids/bases are “weak,”. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow. to review, buffer solutions contain a weak. Explain Buffer Solution In Biology.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Explain Buffer Solution In Biology a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Because these acids/bases are “weak,”. the purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow.. Explain Buffer Solution In Biology.
From www.youtube.com
Buffers and pH Biology YouTube Explain Buffer Solution In Biology ph, acids, bases and buffers | introduction to biology. to review, buffer solutions contain a weak acid and its conjugate base. a biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. By the end. Explain Buffer Solution In Biology.