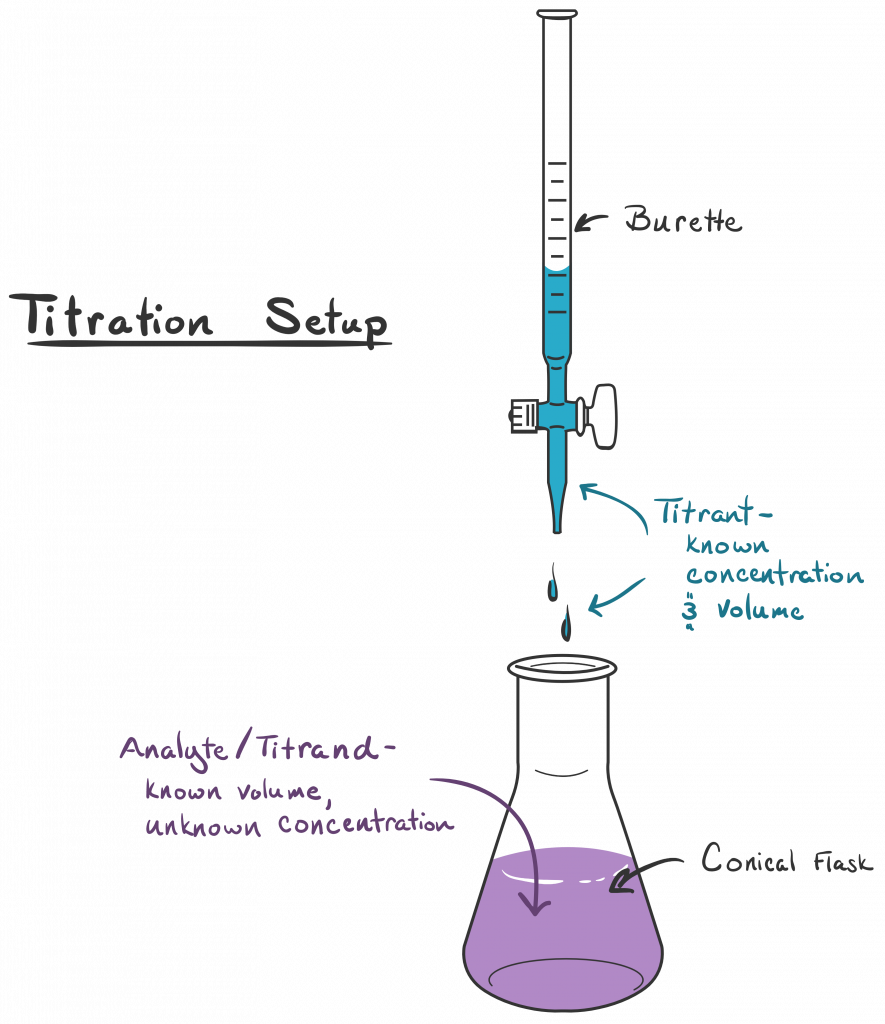
What Is Titration Simple Definition . a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. It is used to work out the unknown concentration of a known substance. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it. titration is a type of quantitative chemical analysis.
from www.microlit.com
titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is the process in which one solution is added to another solution such that it. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. It is used to work out the unknown concentration of a known substance. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration is a type of quantitative chemical analysis. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte.
An Advanced Guide to Titration Microlit
What Is Titration Simple Definition titration is the process in which one solution is added to another solution such that it. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is a type of quantitative chemical analysis. titration is the process in which one solution is added to another solution such that it. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. It is used to work out the unknown concentration of a known substance. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution.
From hxesyopwi.blob.core.windows.net
Titration Definition In Education at Alexandra Bradford blog What Is Titration Simple Definition a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration, process of chemical analysis in which the quantity of some constituent of. What Is Titration Simple Definition.
From chem4three.blogspot.co.uk
CHEMISTRY 11 TITRATIONS What Is Titration Simple Definition titration is the process in which one solution is added to another solution such that it. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is a type of quantitative chemical analysis. This process continues until stoichiometrically equivalent amounts of the reactants have been. What Is Titration Simple Definition.
From gioldvmyt.blob.core.windows.net
Titration Chemical Method at Karl Twiggs blog What Is Titration Simple Definition This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to. What Is Titration Simple Definition.
From www.microlit.com
An Advanced Guide to Titration Microlit What Is Titration Simple Definition a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is the process in which one solution is added to another solution such that it. titration is a type of quantitative chemical analysis. This process continues until stoichiometrically equivalent amounts of the reactants have been. What Is Titration Simple Definition.
From fyoapfhcs.blob.core.windows.net
Titration Lab Simple at Justin Funk blog What Is Titration Simple Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. What Is Titration Simple Definition.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and What Is Titration Simple Definition It is used to work out the unknown concentration of a known substance. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is a type of quantitative chemical analysis. titration is the slow addition of one solution of a known concentration (called a titrant). What Is Titration Simple Definition.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co What Is Titration Simple Definition titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration is the slow addition of. What Is Titration Simple Definition.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis What Is Titration Simple Definition titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which. What Is Titration Simple Definition.
From mmerevise.co.uk
Titrations and Uncertainties MME What Is Titration Simple Definition titration is a type of quantitative chemical analysis. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is. What Is Titration Simple Definition.
From hxelcilfq.blob.core.windows.net
What Is Titration In Chemistry Easy Definition at Kayla Walters blog What Is Titration Simple Definition It is used to work out the unknown concentration of a known substance. titration is a type of quantitative chemical analysis. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is the slow addition of one solution. What Is Titration Simple Definition.
From owlcation.com
What Is Titration? The 3 Types of Titration Explained Owlcation What Is Titration Simple Definition This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. It is used to work out the unknown concentration of a known substance. titration is the process in which one solution is added to another solution such that it. titration is a type of. What Is Titration Simple Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators What Is Titration Simple Definition titration is the process in which one solution is added to another solution such that it. titration is a type of quantitative chemical analysis. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. This process continues until stoichiometrically equivalent amounts of the reactants have been. What Is Titration Simple Definition.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint What Is Titration Simple Definition a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. It is used to work out the unknown concentration of a known substance. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. . What Is Titration Simple Definition.
From theedge.com.hk
Chemistry How To Titration The Edge What Is Titration Simple Definition titration is the process in which one solution is added to another solution such that it. titration is a type of quantitative chemical analysis. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. It is used to work. What Is Titration Simple Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation What Is Titration Simple Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it. titration is a type of quantitative chemical analysis. It is used to work out the unknown concentration of. What Is Titration Simple Definition.
From letitsnowglobe.co.uk
Titration procedure pdf What Is Titration Simple Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. a titration is a technique where a solution of known concentration is. What Is Titration Simple Definition.
From mydigitalkemistry.com
What is Acid Base Titration in Simple Words? What Is Titration Simple Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a. What Is Titration Simple Definition.
From mungfali.com
Titration Steps What Is Titration Simple Definition titration is a type of quantitative chemical analysis. It is used to work out the unknown concentration of a known substance. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration, process of chemical analysis in which the quantity of some constituent of. What Is Titration Simple Definition.
From worksheetcampusslewed.z21.web.core.windows.net
How To Do Titrations In Chemistry What Is Titration Simple Definition It is used to work out the unknown concentration of a known substance. titration is a type of quantitative chemical analysis. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint. What Is Titration Simple Definition.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs What Is Titration Simple Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. It is used to work out the unknown concentration of a known substance. titration is the process. What Is Titration Simple Definition.
From thechemistrynotes.com
Acidbase Titration 4 Types, Theory, Working Principle What Is Titration Simple Definition It is used to work out the unknown concentration of a known substance. titration is a type of quantitative chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose. What Is Titration Simple Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple What Is Titration Simple Definition titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. titration is a type of quantitative chemical analysis. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution.. What Is Titration Simple Definition.
From www.youtube.com
titration part1 definition YouTube What Is Titration Simple Definition titration is the process in which one solution is added to another solution such that it. It is used to work out the unknown concentration of a known substance. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration, process of chemical analysis. What Is Titration Simple Definition.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation What Is Titration Simple Definition titration is the process in which one solution is added to another solution such that it. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the. What Is Titration Simple Definition.
From exyjmshzl.blob.core.windows.net
La Titration Definition at Abraham Godsey blog What Is Titration Simple Definition titration is the process in which one solution is added to another solution such that it. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the. What Is Titration Simple Definition.
From bramblechemistry.weebly.com
4C6 Titration What Is Titration Simple Definition This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is a type of quantitative chemical analysis. titration is the process in which one solution. What Is Titration Simple Definition.
From www.sliderbase.com
Titration What Is Titration Simple Definition titration is the process in which one solution is added to another solution such that it. It is used to work out the unknown concentration of a known substance. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. . What Is Titration Simple Definition.
From www.youtube.com
Titration Definition, steps and formula YouTube What Is Titration Simple Definition titration is a type of quantitative chemical analysis. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the analyte. It is used to. What Is Titration Simple Definition.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry What Is Titration Simple Definition This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration is a type of quantitative chemical analysis. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is. What Is Titration Simple Definition.
From school.careers360.com
types of titration Overview, Structure, Properties & Uses What Is Titration Simple Definition It is used to work out the unknown concentration of a known substance. titration is the process in which one solution is added to another solution such that it. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration is the slow addition. What Is Titration Simple Definition.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube What Is Titration Simple Definition titration is a type of quantitative chemical analysis. It is used to work out the unknown concentration of a known substance. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration is the slow addition of one solution of a known concentration (called. What Is Titration Simple Definition.
From gamesmartz.com
Titration Definition & Image GameSmartz What Is Titration Simple Definition titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. It is used to work out the unknown concentration of a known substance. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is a type of quantitative. What Is Titration Simple Definition.
From fyoxsyypa.blob.core.windows.net
In Titration What Goes In Burette Acid Or Base at Douglas Cox blog What Is Titration Simple Definition titration is a type of quantitative chemical analysis. This process continues until stoichiometrically equivalent amounts of the reactants have been mixed, and an endpoint known as the equivalence point has been reached. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is used. What Is Titration Simple Definition.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool What Is Titration Simple Definition titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is used to work out the unknown concentration of a known substance. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to. What Is Titration Simple Definition.
From scienceinfo.com
Titration Definition, 4 Types, Procedure What Is Titration Simple Definition a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is used to work out the unknown concentration of a known substance. . What Is Titration Simple Definition.