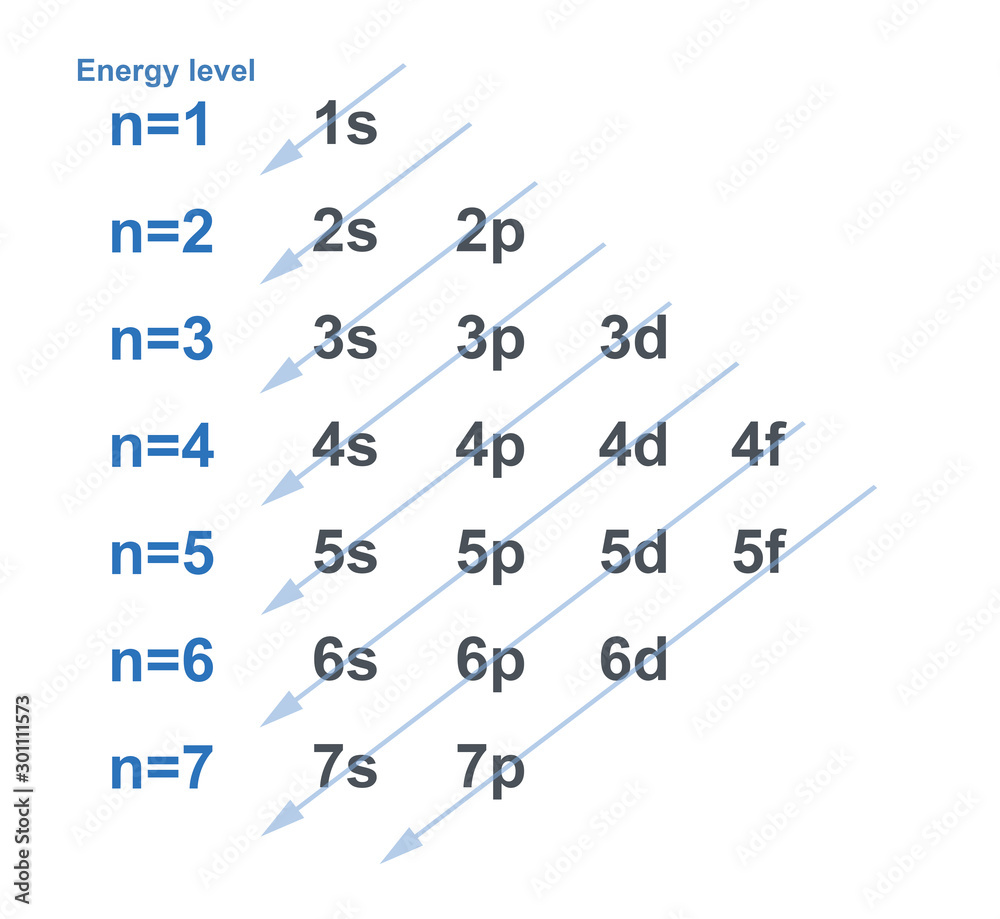
Copper And Chromium Electron Configuration A Level . To understand why this occurs, it is important to. Cr = [ar] 4s1 3d5. Find out how ionisation energies vary with effective. Learn about electron configurations, complexes,. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. The actual electron configurations are: See examples, exceptions and practice. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Cu = [ar] 4s1 3d10. Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. Chromium and copper have the following electron configurations, which are different to what you may expect:
from stock.adobe.com
Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. Learn about electron configurations, complexes,. To understand why this occurs, it is important to. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Cr = [ar] 4s1 3d5. Chromium and copper have the following electron configurations, which are different to what you may expect: Find out how ionisation energies vary with effective. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. See examples, exceptions and practice.
chart of electron configuration with each energy level for element in
Copper And Chromium Electron Configuration A Level Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Chromium and copper have the following electron configurations, which are different to what you may expect: Cr = [ar] 4s1 3d5. To understand why this occurs, it is important to. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Learn about electron configurations, complexes,. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. The actual electron configurations are: Cu = [ar] 4s1 3d10. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Find out how ionisation energies vary with effective. Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. See examples, exceptions and practice.
From slideplayer.com
Electron configuration exceptions. There are several exceptions to the Copper And Chromium Electron Configuration A Level Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. To understand why this occurs, it is important to. Cu = [ar] 4s1 3d10. See examples, exceptions and practice. Copper and chromium are exceptions to the rule. Copper And Chromium Electron Configuration A Level.
From schematicfixpulpits.z21.web.core.windows.net
Electron Configuration Energy Level Diagram Copper And Chromium Electron Configuration A Level The actual electron configurations are: Learn about electron configurations, complexes,. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. To understand why this occurs, it is important to. Learn how to write electron configurations for transition. Copper And Chromium Electron Configuration A Level.
From higher-secondary-chemistry.blogspot.com
Higher Secondary Chemistry Chapter 2.19 Electronic configuration Copper And Chromium Electron Configuration A Level The actual electron configurations are: Learn about electron configurations, complexes,. Chromium and copper have the following electron configurations, which are different to what you may expect: Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Cu = [ar] 4s1 3d10. Learn how to write electron configurations for transition metals, including copper,. Copper And Chromium Electron Configuration A Level.
From carisca.github.io
Electron Configuration Of Chromium Chromium Atomic Electron Copper And Chromium Electron Configuration A Level Cr = [ar] 4s1 3d5. To understand why this occurs, it is important to. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Cu = [ar] 4s1 3d10. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. The actual electron configurations are: Learn how electrons. Copper And Chromium Electron Configuration A Level.
From animalia-life.club
Chromium Orbital Diagram Copper And Chromium Electron Configuration A Level Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Cr = [ar] 4s1 3d5. See examples, exceptions and practice. The actual electron configurations are: Chromium and copper have the following electron configurations, which are different to what you may expect: Copper and chromium are exceptions to the rule. Copper And Chromium Electron Configuration A Level.
From www.pinterest.com.au
Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules Copper And Chromium Electron Configuration A Level Learn about electron configurations, complexes,. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Cr = [ar] 4s1 3d5. To understand why this occurs, it is important to. Cu = [ar] 4s1 3d10. Copper and chromium are exceptions to the rule that the 4s subshell is filled before. Copper And Chromium Electron Configuration A Level.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper And Chromium Electron Configuration A Level Find out how ionisation energies vary with effective. See examples, exceptions and practice. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. The actual electron configurations are: Cu = [ar] 4s1 3d10. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the. Copper And Chromium Electron Configuration A Level.
From stock.adobe.com
chart of electron configuration with each energy level for element in Copper And Chromium Electron Configuration A Level Find out how ionisation energies vary with effective. The actual electron configurations are: Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. See examples, exceptions and practice. Cr = [ar] 4s1 3d5. Cu = [ar] 4s1. Copper And Chromium Electron Configuration A Level.
From ecurrencythailand.com
What Is The Electronic Configuration Of Chromium Z Is Equal To 24? The Copper And Chromium Electron Configuration A Level See examples, exceptions and practice. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Learn about electron configurations, complexes,. Copper and chromium are exceptions to the rule. Copper And Chromium Electron Configuration A Level.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Copper And Chromium Electron Configuration A Level Chromium and copper have the following electron configurations, which are different to what you may expect: Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Cr is [ar] 3d 5. Copper And Chromium Electron Configuration A Level.
From ar.inspiredpencil.com
Chromium Electron Configuration Copper And Chromium Electron Configuration A Level See examples, exceptions and practice. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. Chromium and copper have the following electron configurations, which are different to what you may expect: The actual electron. Copper And Chromium Electron Configuration A Level.
From diagramacademy.com
Electron Configuration of Chromium Diagram Copper And Chromium Electron Configuration A Level Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Cu = [ar] 4s1 3d10. Chromium and copper have the following electron configurations, which are different to what you may expect: See examples, exceptions. Copper And Chromium Electron Configuration A Level.
From www.doubtnut.com
Doubt Solutions Maths, Science, CBSE, NCERT, IIT JEE, NEET Copper And Chromium Electron Configuration A Level Find out how ionisation energies vary with effective. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Learn about electron configurations, complexes,. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Cu = [ar] 4s1 3d10. Learn how to. Copper And Chromium Electron Configuration A Level.
From schematicmaxeywheezle.z21.web.core.windows.net
Orbital Energy Diagram For Copper Copper And Chromium Electron Configuration A Level The actual electron configurations are: Cu = [ar] 4s1 3d10. Chromium and copper have the following electron configurations, which are different to what you may expect: Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. To. Copper And Chromium Electron Configuration A Level.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper And Chromium Electron Configuration A Level Cr = [ar] 4s1 3d5. Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. Cu = [ar] 4s1 3d10. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Chromium and copper have the following electron configurations, which are different to what you. Copper And Chromium Electron Configuration A Level.
From www.toppr.com
The electronic configurations of 24Cr and 29Cu are abnormal Copper And Chromium Electron Configuration A Level Learn about electron configurations, complexes,. The actual electron configurations are: Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Cr = [ar] 4s1 3d5. Find out how ionisation energies vary with effective. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. See examples, exceptions and. Copper And Chromium Electron Configuration A Level.
From www.youtube.com
Electronic configuration of chromium and copper called exceptions Copper And Chromium Electron Configuration A Level Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Find out how ionisation energies vary with effective. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Learn how electrons fill orbitals and energy levels according to the rules of. Copper And Chromium Electron Configuration A Level.
From www.youtube.com
ELECTRONIC CONFIGURATION OF COPPER ATOM and STABILITY YouTube Copper And Chromium Electron Configuration A Level Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. See examples, exceptions and practice. Find out how ionisation energies vary with effective. Chromium and copper have the following electron configurations, which are different to what you may expect: Cr is [ar] 3d 5 4s 1 not [ar] 3d. Copper And Chromium Electron Configuration A Level.
From www.animalia-life.club
Electron Configuration Copper And Chromium Electron Configuration A Level Learn about electron configurations, complexes,. Chromium and copper have the following electron configurations, which are different to what you may expect: The actual electron configurations are: See examples, exceptions and practice. Find out how ionisation energies vary with effective. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d.. Copper And Chromium Electron Configuration A Level.
From www.youtube.com
Electron Configuration Exceptions, Chromium and Copper Revison for A Copper And Chromium Electron Configuration A Level Cu = [ar] 4s1 3d10. To understand why this occurs, it is important to. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. The actual electron configurations are: Chromium and. Copper And Chromium Electron Configuration A Level.
From www.youtube.com
How to write/find/do the electron configuration of Cr(Chromium) and Cu Copper And Chromium Electron Configuration A Level Learn about electron configurations, complexes,. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Cu = [ar] 4s1 3d10. The actual electron configurations are: Find out how ionisation energies vary. Copper And Chromium Electron Configuration A Level.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper And Chromium Electron Configuration A Level Learn about electron configurations, complexes,. Cu = [ar] 4s1 3d10. Cr = [ar] 4s1 3d5. To understand why this occurs, it is important to. See examples, exceptions and practice. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. The actual electron configurations are: Copper and chromium are exceptions. Copper And Chromium Electron Configuration A Level.
From animalia-life.club
Chromium Orbital Diagram Copper And Chromium Electron Configuration A Level Find out how ionisation energies vary with effective. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Cr = [ar] 4s1 3d5. Cu = [ar] 4s1 3d10. The actual electron configurations are: Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. To understand why this. Copper And Chromium Electron Configuration A Level.
From www.alamy.com
Chromium (Cr). Diagram of the nuclear composition and electron Copper And Chromium Electron Configuration A Level Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. Chromium and copper have the following electron configurations, which are different to what you may expect: Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half. Copper And Chromium Electron Configuration A Level.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper And Chromium Electron Configuration A Level The actual electron configurations are: Find out how ionisation energies vary with effective. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Copper and chromium are exceptions to the rule that the 4s subshell is filled. Copper And Chromium Electron Configuration A Level.
From byjus.com
what is electronic configuration of copper and iron Copper And Chromium Electron Configuration A Level Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. To understand why this occurs, it is important to. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Chromium and copper have the following electron configurations, which are different to. Copper And Chromium Electron Configuration A Level.
From www.slideserve.com
PPT Electronic Configuration PowerPoint Presentation ID4748754 Copper And Chromium Electron Configuration A Level Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Learn about electron configurations, complexes,. To understand why this occurs, it is important to. See examples, exceptions and practice. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Find out how ionisation energies vary with effective.. Copper And Chromium Electron Configuration A Level.
From slideplayer.com
Electronic Structure of Atoms ppt download Copper And Chromium Electron Configuration A Level Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Cr = [ar] 4s1 3d5. Find out how ionisation energies vary with effective. Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. Chromium and copper have the following electron configurations, which are different to what you. Copper And Chromium Electron Configuration A Level.
From noredsheet.weebly.com
Chromium electron configuration noredsheet Copper And Chromium Electron Configuration A Level Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Chromium and copper have the following electron configurations, which are different to what you may expect: Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. See examples, exceptions and practice. Cr is [ar]. Copper And Chromium Electron Configuration A Level.
From www.youtube.com
Chromium (Cr) Electron Configuration YouTube Copper And Chromium Electron Configuration A Level Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. The actual electron configurations are: To understand why this occurs, it is important to. Find out how ionisation energies vary with. Copper And Chromium Electron Configuration A Level.
From slideplayer.com
Electron Configurations ppt download Copper And Chromium Electron Configuration A Level Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Cr = [ar] 4s1 3d5. Learn how to write electron configurations for transition metals, including copper, using the periodic table and the order of filling orbitals. See examples, exceptions and practice. Chromium and copper have the following electron configurations, which are different to what you may expect: Learn. Copper And Chromium Electron Configuration A Level.
From www.alamy.com
Symbol and electron diagram for Copper illustration Stock Vector Image Copper And Chromium Electron Configuration A Level Find out how ionisation energies vary with effective. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Chromium and copper have the following electron configurations, which are different to what you may expect: Cr = [ar] 4s1 3d5. Learn how electrons fill orbitals and energy levels according to the rules of. Copper And Chromium Electron Configuration A Level.
From www.youtube.com
Exceptions to Aufbau principle Chromium and Copper configuration BSc Copper And Chromium Electron Configuration A Level To understand why this occurs, it is important to. Cr = [ar] 4s1 3d5. The actual electron configurations are: Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Learn about electron configurations, complexes,. See examples, exceptions and practice. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Chromium. Copper And Chromium Electron Configuration A Level.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper And Chromium Electron Configuration A Level To understand why this occurs, it is important to. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Copper and chromium are exceptions to the rule that the 4s subshell is filled before the 3d subshell. Learn how electrons fill orbitals and energy levels according to the rules. Copper And Chromium Electron Configuration A Level.
From ar.inspiredpencil.com
Electron Configuration Of Chromium Copper And Chromium Electron Configuration A Level Cu = [ar] 4s1 3d10. Learn how electrons fill orbitals and energy levels according to the rules of quantum mechanics. Chromium and copper are two examples of elements with electronic arrangements that show electrons favouring the half or fully filled d. Cr is [ar] 3d 5 4s 1 not [ar] 3d 4 4s. Learn how to write electron configurations for. Copper And Chromium Electron Configuration A Level.