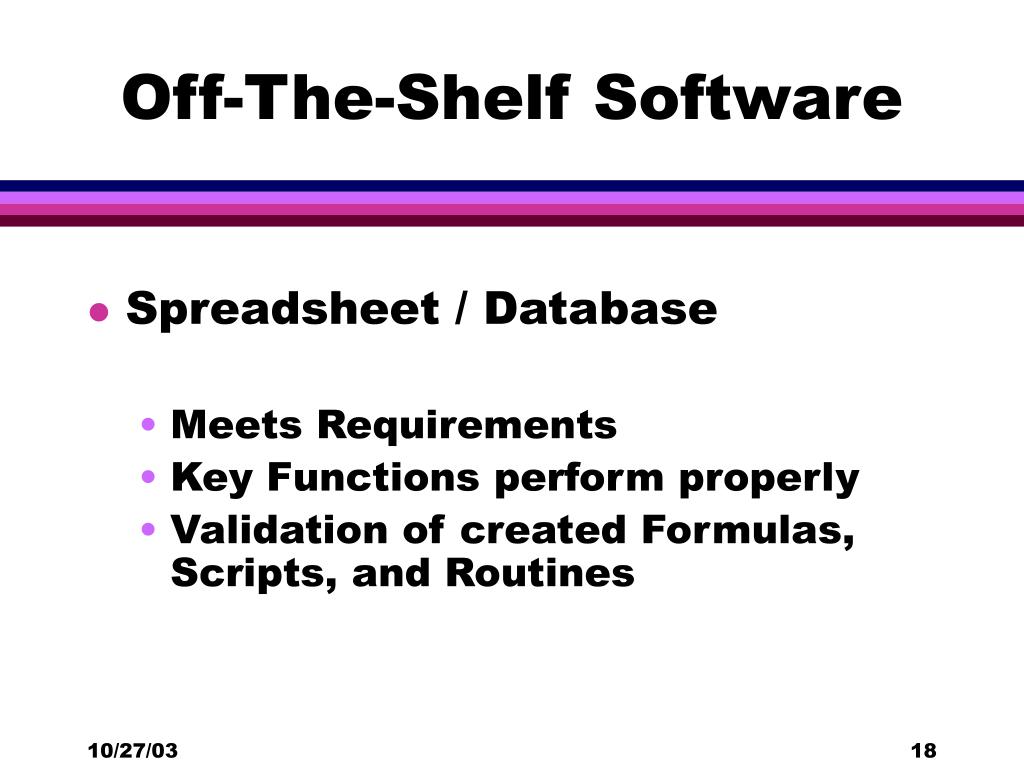
From fyodwgbze.blob.core.windows.net
Off The Shelf Software Bbc Bitesize at Herbert Gritton blog Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From www.mavensolutions.tech
How does offtheshelf software hinder growth? Maven Solutions Off The Shelf Software Fda This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From joifotpvz.blob.core.windows.net
Commercial Off The Shelf Software S Are Also Called at David Matthews blog Off The Shelf Software Fda Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Off The Shelf Software Fda.
From diceus.com
Custom vs OfftheShelf Software Advantages & Disadvantages Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From moreapp.com
OfftheShelf Software vs Custom Software MoreApp Blog Off The Shelf Software Fda This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From www.regdesk.co
FDA Revised Guidance on OffTheShelf Software Use Introduction RegDesk Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From exocfgweo.blob.core.windows.net
One Advantage Of Proprietary Software Versus OffTheShelf Software Is Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From www.slideserve.com
PPT FDA Expectations for Validation of Computer Systems PowerPoint Off The Shelf Software Fda Learn how to document ots software used in a medical device for fda review. This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From www.valuecoders.com
Custom Software vs. OfftheShelf Startup Choices Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. This guidance provides recommendations on software. Off The Shelf Software Fda.
From decode.agency
Choosing between custom vs. offtheshelf software Off The Shelf Software Fda This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From innolitics.com
2019 FDA Guidance OffTheShelf Software Use in Medical Devices Off The Shelf Software Fda This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From www.viftech.com
Custom Software vs OfftheShelf Software What's the Difference? Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From joifotpvz.blob.core.windows.net
Commercial Off The Shelf Software S Are Also Called at David Matthews blog Off The Shelf Software Fda Learn how to document ots software used in a medical device for fda review. This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From giofhehki.blob.core.windows.net
OffTheShelf Software Definition at Jacob Pettit blog Off The Shelf Software Fda Learn how to document ots software used in a medical device for fda review. This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From giofhehki.blob.core.windows.net
OffTheShelf Software Definition at Jacob Pettit blog Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From www.weetechsolution.com
10 Best OfftheShelf Software Examples Ready to Transform Your Business Off The Shelf Software Fda This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From www.studocu.com
OffTheShelf Software Use in Medical Devices Aug 2023 OffTheShelf Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From morioh.com
Custom Software vs Offtheshelf Software How to select a better one Off The Shelf Software Fda Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Off The Shelf Software Fda.
From www.regdesk.co
FDA Revised Guidance on OffTheShelf Software Risk Assessment and Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From www.sgs.com.tw
FDA 最新指引:醫療設備中第三方OTS軟體 (Offtheshelf ) SGS 台灣 Off The Shelf Software Fda This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From www.regdesk.co
FDA Guidance on OffTheShelf Software Use in Medical Devices RegDesk Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From congenius.ch
OffTheShelf software in medical devices FDA guidance Congenius Off The Shelf Software Fda This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From www.complianceonline.com
Guidance for OfftheShelf Software Use in Medical Devices Off The Shelf Software Fda This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From www.thirdrocktechkno.com
Custom vs Offtheshelf software solution A Comparative Guide Off The Shelf Software Fda Learn how to document ots software used in a medical device for fda review. This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From www.slideserve.com
PPT FDA Expectations for Validation of Computer Systems PowerPoint Off The Shelf Software Fda This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From fyolfqnhg.blob.core.windows.net
Off The Shelf Software General Purpose at Tammy Johnson blog Off The Shelf Software Fda This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From itechnolabs.ca
What is a COTS Software [Updated 2024] Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. Off The Shelf Software Fda.
From exoungkyt.blob.core.windows.net
OffTheShelf Software Solutions at Rose Benson blog Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. This guidance provides recommendations on software. Off The Shelf Software Fda.
From innolitics.com
2019 FDA Guidance OffTheShelf Software Use in Medical Devices Off The Shelf Software Fda The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Learn how to document ots software used in a medical device for fda review. This guidance provides recommendations on software. Off The Shelf Software Fda.
From xbsoftware.com
Custom Software vs OfftheShelf Software XB Software Off The Shelf Software Fda Learn how to document ots software used in a medical device for fda review. This guidance provides recommendations on software. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From invozone.com
Why Should and Shouldn’t I Use Off the Shelf Software? Off The Shelf Software Fda Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Off The Shelf Software Fda.
From www.regdesk.co
FDA Revised Guidance on OffTheShelf Software Use Maintenance and Off The Shelf Software Fda This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From simpat.tech
5 OffTheShelf Software Advantages and Disadvantages Simpat Tech Off The Shelf Software Fda Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. This guidance provides recommendations on software. Off The Shelf Software Fda.
From innolitics.com
OffTheShelf Software Best Practices, FAQs, and Examples Off The Shelf Software Fda This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.
From www.regdesk.co
FDA Guidance on OffTheShelf Software Use in Medical Devices RegDesk Off The Shelf Software Fda This guidance provides recommendations on software. Learn how to document ots software used in a medical device for fda review. The guidance covers topics such as software description, risk assessment, testing, changes, and labeling. Off The Shelf Software Fda.