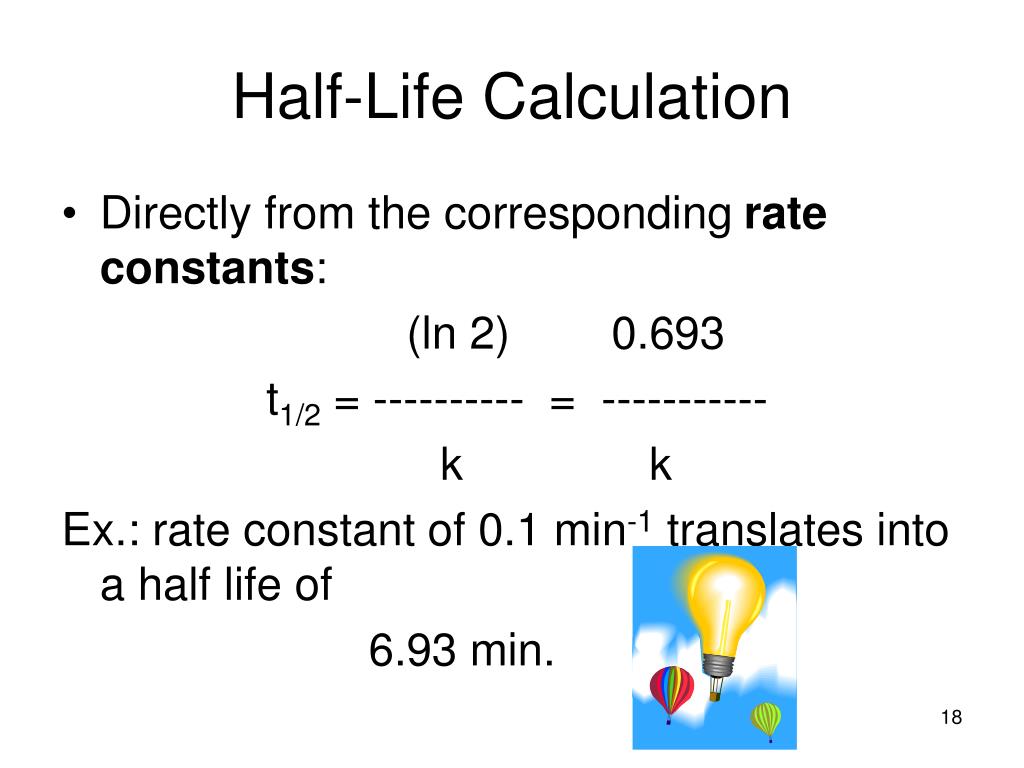
Half Life Equation Rate Constant . We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The initial rates and the rate equation; Calculating the rate constant from the. The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: By dividing 0.693 by the.
from www.slideserve.com
The order of the reaction or enough information to. The initial rates and the rate equation; By dividing 0.693 by the. The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order.
PPT Concepts of Pharmacology Half Life Calculation PowerPoint Presentation ID307737
Half Life Equation Rate Constant The initial rates and the rate equation; By dividing 0.693 by the. The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; Calculating the rate constant from the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order.
From www.chegg.com
Solved Define the terms rate constant k and half life Half Life Equation Rate Constant The initial rates and the rate equation; By dividing 0.693 by the. The rate constant (k) of a reaction can be calculated using: We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The order of the reaction or enough information to. Calculating the rate constant from the. Half Life Equation Rate Constant.
From www.slideserve.com
PPT Chemical Chapter 14 PowerPoint Presentation, free download ID4206707 Half Life Equation Rate Constant The order of the reaction or enough information to. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The initial rates and the rate equation; By dividing 0.693 by the. Calculating the rate constant from the. The rate constant (k) of a reaction can be calculated using: Half Life Equation Rate Constant.
From www.numerade.com
SOLVED The halflife for the zero order reaction A → Products is 276 s. What is the value of Half Life Equation Rate Constant The rate constant (k) of a reaction can be calculated using: By dividing 0.693 by the. The order of the reaction or enough information to. Calculating the rate constant from the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The initial rates and the rate equation; Half Life Equation Rate Constant.
From www.youtube.com
The First Order Integrated Rate Law and Half Life (Part 4) YouTube Half Life Equation Rate Constant The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; Calculating the rate constant from the. By dividing 0.693 by the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The order of the reaction or enough information to. Half Life Equation Rate Constant.
From www.slideserve.com
PPT Concepts of Pharmacology Half Life Calculation PowerPoint Presentation ID307737 Half Life Equation Rate Constant We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. By dividing 0.693 by the. Calculating the rate constant from the. The order of the reaction or enough information to. The initial rates and the rate equation; The rate constant (k) of a reaction can be calculated using: Half Life Equation Rate Constant.
From haipernews.com
How To Calculate Half Life Reaction Haiper Half Life Equation Rate Constant The initial rates and the rate equation; Calculating the rate constant from the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The rate constant (k) of a reaction can be calculated using: The order of the reaction or enough information to. By dividing 0.693 by the. Half Life Equation Rate Constant.
From www.youtube.com
integrated rate law and halflife expression for general Nth order reaction, N ≠ 1 Half Life Equation Rate Constant We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; Calculating the rate constant from the. By dividing 0.693 by the. Half Life Equation Rate Constant.
From www.coursehero.com
[Solved] Derive the integrated rate law and the halflife equations for the... Course Hero Half Life Equation Rate Constant Calculating the rate constant from the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The rate constant (k) of a reaction can be calculated using: By dividing 0.693 by the. The order of the reaction or enough information to. The initial rates and the rate equation; Half Life Equation Rate Constant.
From www.youtube.com
5.3 Rate Constant & Half Life YouTube Half Life Equation Rate Constant The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. The order of the reaction or enough information to. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The initial rates and the rate equation; By dividing 0.693 by the. Half Life Equation Rate Constant.
From dxozpbads.blob.core.windows.net
Half Life Balanced Equation at Mark Watts blog Half Life Equation Rate Constant The initial rates and the rate equation; The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. By dividing 0.693 by the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The order of the reaction or enough information to. Half Life Equation Rate Constant.
From www.researchgate.net
Inactivation Rate Constant (k in ) and Halflife of the Download Table Half Life Equation Rate Constant The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. By dividing 0.693 by the. The initial rates and the rate equation; We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The order of the reaction or enough information to. Half Life Equation Rate Constant.
From trixalagom.blogspot.com
half life formula for first order reaction Burdensome Online Journal Custom Image Library Half Life Equation Rate Constant The rate constant (k) of a reaction can be calculated using: We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The order of the reaction or enough information to. The initial rates and the rate equation; Calculating the rate constant from the. By dividing 0.693 by the. Half Life Equation Rate Constant.
From www.youtube.com
Half Life and Rate Constant of 1st Order Reaction YouTube Half Life Equation Rate Constant We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The initial rates and the rate equation; The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. By dividing 0.693 by the. Half Life Equation Rate Constant.
From www.youtube.com
First Order Elimination Rate Constant and Halflife A closer look Lect 11 YouTube Half Life Equation Rate Constant The initial rates and the rate equation; By dividing 0.693 by the. Calculating the rate constant from the. The rate constant (k) of a reaction can be calculated using: The order of the reaction or enough information to. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. Half Life Equation Rate Constant.
From dxozpbads.blob.core.windows.net
Half Life Balanced Equation at Mark Watts blog Half Life Equation Rate Constant The order of the reaction or enough information to. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; Calculating the rate constant from the. By dividing 0.693 by the. Half Life Equation Rate Constant.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Half Life Equation Rate Constant By dividing 0.693 by the. The initial rates and the rate equation; The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. Half Life Equation Rate Constant.
From www.expii.com
HalfLife — Definition & Overview Expii Half Life Equation Rate Constant The initial rates and the rate equation; By dividing 0.693 by the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. The order of the reaction or enough information to. Half Life Equation Rate Constant.
From haipernews.com
How To Calculate Half Life Algebra Haiper Half Life Equation Rate Constant The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; Calculating the rate constant from the. By dividing 0.693 by the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. Half Life Equation Rate Constant.
From lauranfryer.blogspot.com
half life formula for first order reaction Lauran Fryer Half Life Equation Rate Constant The rate constant (k) of a reaction can be calculated using: The order of the reaction or enough information to. The initial rates and the rate equation; We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. By dividing 0.693 by the. Calculating the rate constant from the. Half Life Equation Rate Constant.
From www.slideserve.com
PPT Concepts of Pharmacology Half Life Calculation PowerPoint Presentation ID307737 Half Life Equation Rate Constant We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The initial rates and the rate equation; By dividing 0.693 by the. The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. Half Life Equation Rate Constant.
From www.toppr.com
Derive the relationship between half life period and rate constant of a zero order reaction. Half Life Equation Rate Constant The initial rates and the rate equation; We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The rate constant (k) of a reaction can be calculated using: The order of the reaction or enough information to. By dividing 0.693 by the. Calculating the rate constant from the. Half Life Equation Rate Constant.
From warreninstitute.org
Master The HALFLIFE Equation Derivation Dive Deeper! Half Life Equation Rate Constant Calculating the rate constant from the. By dividing 0.693 by the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; The order of the reaction or enough information to. Half Life Equation Rate Constant.
From general.chemistrysteps.com
SecondOrder Reactions Chemistry Steps Half Life Equation Rate Constant The initial rates and the rate equation; We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. By dividing 0.693 by the. The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. Half Life Equation Rate Constant.
From yoursoundboard.blogspot.com
half life formula for first order reaction Ashlee Manley Half Life Equation Rate Constant The initial rates and the rate equation; By dividing 0.693 by the. Calculating the rate constant from the. The rate constant (k) of a reaction can be calculated using: We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The order of the reaction or enough information to. Half Life Equation Rate Constant.
From study.com
Identifying HalfLife Given the Rate Constant Chemistry Half Life Equation Rate Constant The order of the reaction or enough information to. Calculating the rate constant from the. The initial rates and the rate equation; The rate constant (k) of a reaction can be calculated using: By dividing 0.693 by the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. Half Life Equation Rate Constant.
From www.youtube.com
Second Order Integrated Rate Law and Half Life (Part 5) YouTube Half Life Equation Rate Constant The order of the reaction or enough information to. By dividing 0.693 by the. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. Calculating the rate constant from the. Half Life Equation Rate Constant.
From ar.inspiredpencil.com
2nd Order Reaction Equation Half Life Equation Rate Constant By dividing 0.693 by the. The rate constant (k) of a reaction can be calculated using: We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. Calculating the rate constant from the. The initial rates and the rate equation; The order of the reaction or enough information to. Half Life Equation Rate Constant.
From www.wikihow.com
How to Calculate Half Life 6 Steps (with Pictures) wikiHow Half Life Equation Rate Constant By dividing 0.693 by the. The order of the reaction or enough information to. Calculating the rate constant from the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; Half Life Equation Rate Constant.
From www.youtube.com
Half Life Equation Derivation YouTube Half Life Equation Rate Constant Calculating the rate constant from the. By dividing 0.693 by the. The initial rates and the rate equation; The rate constant (k) of a reaction can be calculated using: We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The order of the reaction or enough information to. Half Life Equation Rate Constant.
From chemistnotes.com
Half life Formula Definition, and Wellderived equation Chemistry Notes Half Life Equation Rate Constant We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. By dividing 0.693 by the. The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: Calculating the rate constant from the. The initial rates and the rate equation; Half Life Equation Rate Constant.
From www.numerade.com
SOLVED Halflife equation for firstorder reactions t1/2 = 0.693/k where t1/2 is the halflife Half Life Equation Rate Constant The order of the reaction or enough information to. The initial rates and the rate equation; Calculating the rate constant from the. The rate constant (k) of a reaction can be calculated using: By dividing 0.693 by the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. Half Life Equation Rate Constant.
From www.youtube.com
Aleks Finding half life and rate constant from a graph of concentration versus time YouTube Half Life Equation Rate Constant The initial rates and the rate equation; By dividing 0.693 by the. The rate constant (k) of a reaction can be calculated using: The order of the reaction or enough information to. Calculating the rate constant from the. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. Half Life Equation Rate Constant.
From www.youtube.com
13.15 How to find decay constant and half life YouTube Half Life Equation Rate Constant By dividing 0.693 by the. The order of the reaction or enough information to. We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. Calculating the rate constant from the. The rate constant (k) of a reaction can be calculated using: The initial rates and the rate equation; Half Life Equation Rate Constant.
From lauranfryer.blogspot.com
half life formula for first order reaction Lauran Fryer Half Life Equation Rate Constant The initial rates and the rate equation; We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The order of the reaction or enough information to. The rate constant (k) of a reaction can be calculated using: By dividing 0.693 by the. Calculating the rate constant from the. Half Life Equation Rate Constant.
From www.youtube.com
16.2f Finding half life and rate constant from a graph of concentration versus time YouTube Half Life Equation Rate Constant Calculating the rate constant from the. By dividing 0.693 by the. The initial rates and the rate equation; We can use integrated rate laws with experimental data that consist of time and concentration information to determine the order. The rate constant (k) of a reaction can be calculated using: The order of the reaction or enough information to. Half Life Equation Rate Constant.