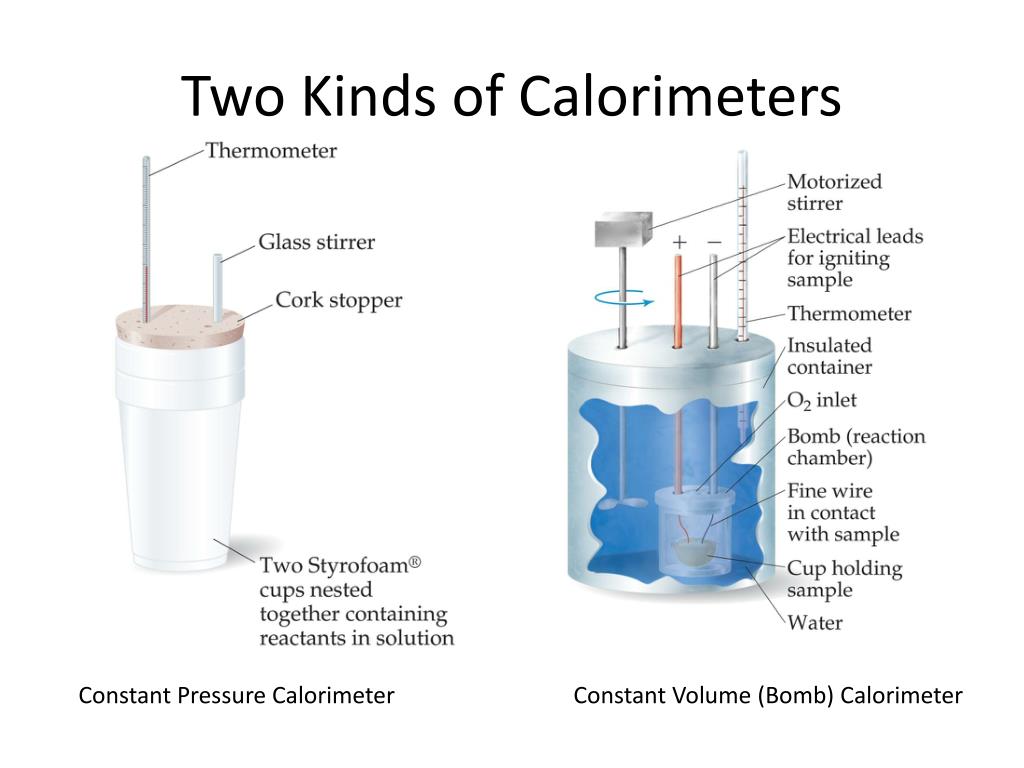
What Does A Calorimeter Measure . Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Learn about different types of calorimeters, the calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Apply the first law of thermodynamics to calorimetry. It uses devices called calorimeters, which measure the change in.
from www.slideserve.com
Apply the first law of thermodynamics to calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the change in. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Learn about different types of calorimeters, the calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction.
PPT Calorimetry PowerPoint Presentation, free download ID3850751
What Does A Calorimeter Measure Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. It uses devices called calorimeters, which measure the change in. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Learn about different types of calorimeters, the calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction.
From www.youtube.com
Constructing a Calorimeter YouTube What Does A Calorimeter Measure Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. What Does A Calorimeter Measure.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide What Does A Calorimeter Measure Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Learn about. What Does A Calorimeter Measure.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson What Does A Calorimeter Measure Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. It uses devices called calorimeters, which measure the change in. Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the heat developed. What Does A Calorimeter Measure.
From thermonine92.blogspot.com
Thermochemistry Calorimeter What Does A Calorimeter Measure Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Apply the first law of thermodynamics to calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimetry is used to measure quantities of heat, and can be used to. What Does A Calorimeter Measure.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? What Does A Calorimeter Measure Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It uses devices called calorimeters, which measure the change in. Learn about different types of calorimeters, the calorimetry. Calorimetry is used to measure quantities of heat, and can. What Does A Calorimeter Measure.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube What Does A Calorimeter Measure Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimetry is. What Does A Calorimeter Measure.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Does A Calorimeter Measure Apply the first law of thermodynamics to calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the set of techniques used to measure enthalpy changes. What Does A Calorimeter Measure.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID3439007 What Does A Calorimeter Measure Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Learn about different types of calorimeters, the. What Does A Calorimeter Measure.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Does A Calorimeter Measure Apply the first law of thermodynamics to calorimetry. Learn about different types of calorimeters, the calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry. What Does A Calorimeter Measure.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Apply the first law of thermodynamics to calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.. What Does A Calorimeter Measure.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry What Does A Calorimeter Measure Learn about different types of calorimeters, the calorimetry. It uses devices called calorimeters, which measure the change in. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is used. What Does A Calorimeter Measure.
From www.animalia-life.club
Calorimeter Diagram What Does A Calorimeter Measure Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is a field of thermochemistry that measures the amount of. What Does A Calorimeter Measure.
From exysipkrj.blob.core.windows.net
How Does A Calorimeter Measure The Heat Content In Food at Carlos Hodge blog What Does A Calorimeter Measure Learn about different types of calorimeters, the calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. It uses devices called calorimeters, which measure the change in. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a. What Does A Calorimeter Measure.
From www.pinterest.com
ConstantPressure Calorimetery "coffeecup" calorimeter often used to measure heat transferred What Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is a field of thermochemistry that measures the amount of. What Does A Calorimeter Measure.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Does A Calorimeter Measure It uses devices called calorimeters, which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Compare heat flow from hot to cold objects in an ideal. What Does A Calorimeter Measure.
From www.youtube.com
Principle of Calorimetry YouTube What Does A Calorimeter Measure Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the set of. What Does A Calorimeter Measure.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Does A Calorimeter Measure A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. It uses devices called calorimeters, which measure the change in. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare heat flow from hot to cold. What Does A Calorimeter Measure.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of What Does A Calorimeter Measure Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. It uses devices called calorimeters, which measure the change in. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Learn about different types of. What Does A Calorimeter Measure.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Does A Calorimeter Measure Learn about different types of calorimeters, the calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the. What Does A Calorimeter Measure.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness What Does A Calorimeter Measure It uses devices called calorimeters, which measure the change in. Learn about different types of calorimeters, the calorimetry. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Apply the first law of thermodynamics to calorimetry. A calorimeter measures the mass of liquid and the temperature change of the. What Does A Calorimeter Measure.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses What Does A Calorimeter Measure Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. It uses devices called calorimeters, which measure the change in. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Compare heat flow from hot to cold objects in an ideal. What Does A Calorimeter Measure.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Does A Calorimeter Measure Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Learn about different types of calorimeters, the calorimetry. Calorimetry is the set of techniques used to. What Does A Calorimeter Measure.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Does A Calorimeter Measure Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Learn about different types of calorimeters, the calorimetry. Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the. What Does A Calorimeter Measure.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Exams What Does A Calorimeter Measure A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Apply the first law of thermodynamics to calorimetry. It uses devices called calorimeters, which measure. What Does A Calorimeter Measure.
From ddscalorimeters.com
A Coffee Cup Calorimeter vs A Bomb Calorimeter DDS What Does A Calorimeter Measure Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Learn about different types of calorimeters, the calorimetry. Apply the first law of thermodynamics. What Does A Calorimeter Measure.
From www.education.com
Calorimetry Bomb Calorimeter Experiment What Does A Calorimeter Measure Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.. What Does A Calorimeter Measure.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience What Does A Calorimeter Measure A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Apply the first law of thermodynamics to calorimetry. Calorimeter, device for measuring the heat. What Does A Calorimeter Measure.
From users.highland.edu
Calorimetry What Does A Calorimeter Measure Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter. What Does A Calorimeter Measure.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation & Application What Does A Calorimeter Measure Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity.. What Does A Calorimeter Measure.
From joiddcrwa.blob.core.windows.net
What Is Calorimetry Example at Donovan blog What Does A Calorimeter Measure Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. It uses devices called calorimeters, which measure the change in. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is the set of techniques used to measure. What Does A Calorimeter Measure.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download ID5118958 What Does A Calorimeter Measure It uses devices called calorimeters, which measure the change in. Learn about different types of calorimeters, the calorimetry. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is a field of. What Does A Calorimeter Measure.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Does A Calorimeter Measure Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Apply the first law of thermodynamics to calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Learn about different types of calorimeters, the calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat. What Does A Calorimeter Measure.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 What Does A Calorimeter Measure Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the change in. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. What Does A Calorimeter Measure.
From saylordotorg.github.io
Calorimetry What Does A Calorimeter Measure Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Calorimetry is a field of thermochemistry. What Does A Calorimeter Measure.
From joiksvxlu.blob.core.windows.net
How Does Calorimeter Constant Work at Stacy Cavallo blog What Does A Calorimeter Measure Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Calorimetry. What Does A Calorimeter Measure.