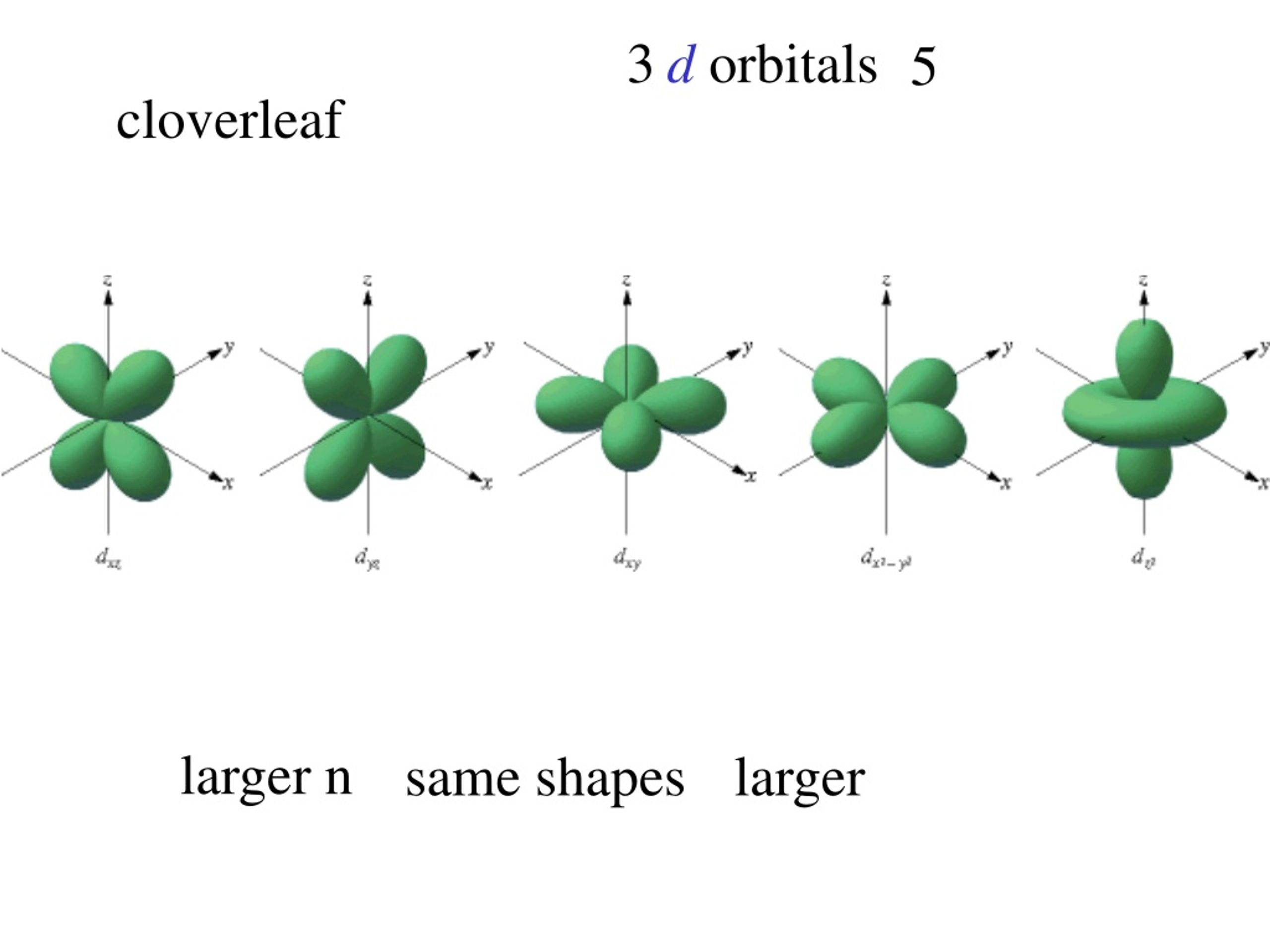
What Is An Orbital In Quantum Mechanics . In hydrogen, there are usually multiple orbitals that share the same energy. We call the solutions “orbitals” rather than just energy levels. An orbital is the quantum mechanical refinement of bohr’s orbit. Thinking about electrons as probabilistic matter waves using the de broglie. An atomic orbital, which is distinct from an. Introduction to the quantum mechanical model of the atom: The principal quantum number is one of three quantum numbers used to characterize an orbital. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability of finding an electron. Shown here is the first balmer transition, in which an electron jumps from. Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived.
from www.slideserve.com
Shown here is the first balmer transition, in which an electron jumps from. In hydrogen, there are usually multiple orbitals that share the same energy. Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability of finding an electron. Introduction to the quantum mechanical model of the atom: The principal quantum number is one of three quantum numbers used to characterize an orbital. We call the solutions “orbitals” rather than just energy levels. An atomic orbital, which is distinct from an. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. An orbital is the quantum mechanical refinement of bohr’s orbit.
PPT Quantum Mechanics and Atomic Orbitals PowerPoint Presentation, free download ID9198702
What Is An Orbital In Quantum Mechanics In hydrogen, there are usually multiple orbitals that share the same energy. In hydrogen, there are usually multiple orbitals that share the same energy. Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. An orbital is the quantum mechanical refinement of bohr’s orbit. Thinking about electrons as probabilistic matter waves using the de broglie. The principal quantum number is one of three quantum numbers used to characterize an orbital. Shown here is the first balmer transition, in which an electron jumps from. We call the solutions “orbitals” rather than just energy levels. An atomic orbital, which is distinct from an. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. Introduction to the quantum mechanical model of the atom: An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability of finding an electron. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus.
From www.expii.com
Quantum Mechanical Model — Overview & History Expii What Is An Orbital In Quantum Mechanics Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanical Model of the Atom PowerPoint Presentation, free download ID5909266 What Is An Orbital In Quantum Mechanics The principal quantum number is one of three quantum numbers used to characterize an orbital. Thinking about electrons as probabilistic matter waves using the de broglie. An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability of finding an electron. Energy is emitted. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanics and Atomic Orbitals PowerPoint Presentation, free download ID9198702 What Is An Orbital In Quantum Mechanics Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. An atomic orbital, which is distinct from an. Shown here is the first balmer transition, in which an electron jumps. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanical Model PowerPoint Presentation, free download ID4977042 What Is An Orbital In Quantum Mechanics An orbital is the quantum mechanical refinement of bohr’s orbit. Shown here is the first balmer transition, in which an electron jumps from. In hydrogen, there are usually multiple orbitals that share the same energy. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. Introduction to the quantum mechanical model of. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanics and Atomic Orbitals PowerPoint Presentation, free download ID9198702 What Is An Orbital In Quantum Mechanics We call the solutions “orbitals” rather than just energy levels. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. An atomic orbital, which is distinct from an. Thinking about electrons as probabilistic matter waves using the de broglie. The principal quantum number is one of three quantum numbers used to. What Is An Orbital In Quantum Mechanics.
From slideplayer.com
Electronic Structure of Atoms ppt download What Is An Orbital In Quantum Mechanics An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability of finding an electron. Shown here is the first balmer transition, in which an electron jumps from. Each wave function with an allowed combination of n, l, and ml values describes an atomic. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanical Model PowerPoint Presentation, free download ID4977042 What Is An Orbital In Quantum Mechanics Introduction to the quantum mechanical model of the atom: Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. An atomic orbital, which is distinct from an. In hydrogen, there are usually multiple orbitals that share the same energy. Energy is emitted from the atom when the electron jumps. What Is An Orbital In Quantum Mechanics.
From chem.libretexts.org
8 The Quantum Hydrogen Atom Chemistry LibreTexts What Is An Orbital In Quantum Mechanics An orbital is the quantum mechanical refinement of bohr’s orbit. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. The principal quantum number is one of three quantum numbers used to characterize an orbital. Thinking about electrons as probabilistic matter waves using the de broglie. In hydrogen, there are usually multiple. What Is An Orbital In Quantum Mechanics.
From en.ppt-online.org
Quantum Mechanics 2 Schroedinger equation. Atomic wave functions. Atomic orbitals. Quantum What Is An Orbital In Quantum Mechanics The principal quantum number is one of three quantum numbers used to characterize an orbital. In hydrogen, there are usually multiple orbitals that share the same energy. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. We call the solutions “orbitals” rather than just energy levels. Thinking about electrons as probabilistic. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanical Model PowerPoint Presentation, free download ID2056835 What Is An Orbital In Quantum Mechanics An orbital is the quantum mechanical refinement of bohr’s orbit. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. An atomic orbital, which is distinct from an. Introduction to the quantum mechanical model of the atom: In hydrogen, there are usually multiple orbitals that share the same energy. Each wave. What Is An Orbital In Quantum Mechanics.
From www.pinterest.com
Orbitals Quantum mechanics, Chemistry, Organic chemistry What Is An Orbital In Quantum Mechanics We call the solutions “orbitals” rather than just energy levels. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. In hydrogen, there are usually multiple orbitals that share the same energy. Thinking. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanics and Atomic Orbitals PowerPoint Presentation, free download ID629965 What Is An Orbital In Quantum Mechanics Thinking about electrons as probabilistic matter waves using the de broglie. An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability of finding an electron. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived.. What Is An Orbital In Quantum Mechanics.
From www.youtube.com
Orbitals and Quantum Mechanics Video 3 YouTube What Is An Orbital In Quantum Mechanics The principal quantum number is one of three quantum numbers used to characterize an orbital. An orbital is the quantum mechanical refinement of bohr’s orbit. Shown here is the first balmer transition, in which an electron jumps from. Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. Introduction. What Is An Orbital In Quantum Mechanics.
From mavink.com
Quantum Numbers And Orbital Shapes What Is An Orbital In Quantum Mechanics Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. The principal quantum number is one of three quantum numbers used to characterize an orbital. We call the solutions “orbitals” rather than just energy levels. Each wave function with an allowed combination of n, l, and ml values describes an atomic. What Is An Orbital In Quantum Mechanics.
From courses.lumenlearning.com
Development of Quantum Theory Chemistry What Is An Orbital In Quantum Mechanics Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. An orbital is the quantum mechanical refinement of bohr’s orbit. An orbital is a mathematical function that describes the matter. What Is An Orbital In Quantum Mechanics.
From www.britannica.com
Orbital Chemistry, Physics & Applications Britannica What Is An Orbital In Quantum Mechanics Introduction to the quantum mechanical model of the atom: Shown here is the first balmer transition, in which an electron jumps from. An orbital is the quantum mechanical refinement of bohr’s orbit. In hydrogen, there are usually multiple orbitals that share the same energy. Energy is emitted from the atom when the electron jumps from one orbit to another closer. What Is An Orbital In Quantum Mechanics.
From www.youtube.com
Chemistry Lesson 9 Quantum Mechanical Model YouTube What Is An Orbital In Quantum Mechanics In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. Thinking about electrons as probabilistic matter waves using the de broglie. We call the solutions “orbitals” rather than just energy levels. Shown here is the first balmer transition, in which an electron jumps from. An atomic orbital, which is distinct from an.. What Is An Orbital In Quantum Mechanics.
From physics.stackexchange.com
quantum mechanics Geometric interpretation of orbital angular momentum eigenvalues Physics What Is An Orbital In Quantum Mechanics The principal quantum number is one of three quantum numbers used to characterize an orbital. Thinking about electrons as probabilistic matter waves using the de broglie. In hydrogen, there are usually multiple orbitals that share the same energy. We call the solutions “orbitals” rather than just energy levels. Energy is emitted from the atom when the electron jumps from one. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanics and Atomic Orbitals PowerPoint Presentation, free download ID629965 What Is An Orbital In Quantum Mechanics We call the solutions “orbitals” rather than just energy levels. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. An atomic orbital, which is distinct from an. Introduction to the quantum mechanical model of the atom: An orbital is a mathematical function that describes the matter wave of an electron —. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID6909895 What Is An Orbital In Quantum Mechanics Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie. Shown here is the first balmer transition, in which an electron jumps from. An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability. What Is An Orbital In Quantum Mechanics.
From www.lancaster.ac.uk
XVII Hydrogen atom‣ Quantum Mechanics — Lecture notes for PHYS223 What Is An Orbital In Quantum Mechanics Shown here is the first balmer transition, in which an electron jumps from. Introduction to the quantum mechanical model of the atom: In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. We call the solutions “orbitals” rather than just energy levels. An orbital is the quantum mechanical refinement of bohr’s orbit.. What Is An Orbital In Quantum Mechanics.
From en.ppt-online.org
Quantum Mechanics 2 Schroedinger equation. Atomic wave functions. Atomic orbitals. Quantum What Is An Orbital In Quantum Mechanics We call the solutions “orbitals” rather than just energy levels. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Thinking about electrons as probabilistic matter waves using the de broglie. Shown here is the first balmer transition, in which an electron jumps from. An orbital is the quantum mechanical refinement. What Is An Orbital In Quantum Mechanics.
From www.shutterstock.com
Atomic Theory Quantum Mechanics Atomic Orbital Stock Vector (Royalty Free) 2019455270 Shutterstock What Is An Orbital In Quantum Mechanics The principal quantum number is one of three quantum numbers used to characterize an orbital. We call the solutions “orbitals” rather than just energy levels. An orbital is the quantum mechanical refinement of bohr’s orbit. An atomic orbital, which is distinct from an. Shown here is the first balmer transition, in which an electron jumps from. Thinking about electrons as. What Is An Orbital In Quantum Mechanics.
From physics.stackexchange.com
quantum mechanics How do orbitals exist in an atom? Physics Stack Exchange What Is An Orbital In Quantum Mechanics In hydrogen, there are usually multiple orbitals that share the same energy. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. Shown here is the first balmer transition, in which an electron jumps from. Introduction to the quantum mechanical model of the atom: Energy is emitted from the atom when the. What Is An Orbital In Quantum Mechanics.
From api-project-1022638073839.appspot.com
What is a set of four quantum numbers that could represent the last electron added (using the What Is An Orbital In Quantum Mechanics An orbital is the quantum mechanical refinement of bohr’s orbit. An atomic orbital, which is distinct from an. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. In hydrogen, there are usually multiple orbitals that share the same energy. Introduction to the quantum mechanical model of the atom: Thinking about electrons. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanics and Atomic Orbitals PowerPoint Presentation, free download ID629965 What Is An Orbital In Quantum Mechanics In hydrogen, there are usually multiple orbitals that share the same energy. An orbital is the quantum mechanical refinement of bohr’s orbit. An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability of finding an electron. Introduction to the quantum mechanical model of. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT The Quantum Model PowerPoint Presentation, free download ID2574252 What Is An Orbital In Quantum Mechanics The principal quantum number is one of three quantum numbers used to characterize an orbital. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. An orbital is the quantum mechanical refinement of bohr’s orbit. An orbital is a mathematical function that describes the matter wave of an electron — it describes. What Is An Orbital In Quantum Mechanics.
From en.ppt-online.org
Quantum Mechanics 2 Schroedinger equation. Atomic wave functions. Atomic orbitals. Quantum What Is An Orbital In Quantum Mechanics Shown here is the first balmer transition, in which an electron jumps from. In hydrogen, there are usually multiple orbitals that share the same energy. Introduction to the quantum mechanical model of the atom: In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. An orbital is a mathematical function that describes. What Is An Orbital In Quantum Mechanics.
From en.ppt-online.org
Quantum Mechanics 2 Schroedinger equation. Atomic wave functions. Atomic orbitals. Quantum What Is An Orbital In Quantum Mechanics An orbital is the quantum mechanical refinement of bohr’s orbit. We call the solutions “orbitals” rather than just energy levels. An atomic orbital, which is distinct from an. Thinking about electrons as probabilistic matter waves using the de broglie. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Shown here. What Is An Orbital In Quantum Mechanics.
From www.dreamstime.com
Atomic Orbital Atomic Theory and Quantum Mechanics, Stock Illustration Illustration of element What Is An Orbital In Quantum Mechanics Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically derived. Shown here is the first balmer transition, in which an electron jumps from. We call the solutions “orbitals” rather than just. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanics and Atomic Orbitals PowerPoint Presentation, free download ID629965 What Is An Orbital In Quantum Mechanics We call the solutions “orbitals” rather than just energy levels. In hydrogen, there are usually multiple orbitals that share the same energy. An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability of finding an electron. Thinking about electrons as probabilistic matter waves. What Is An Orbital In Quantum Mechanics.
From en.ppt-online.org
Quantum Mechanics 2 Schroedinger equation. Atomic wave functions. Atomic orbitals. Quantum What Is An Orbital In Quantum Mechanics Thinking about electrons as probabilistic matter waves using the de broglie. We call the solutions “orbitals” rather than just energy levels. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Introduction to the quantum mechanical model of the atom: An atomic orbital, which is distinct from an. The principal quantum. What Is An Orbital In Quantum Mechanics.
From www.slideserve.com
PPT Quantum Mechanics and Atomic Orbitals PowerPoint Presentation, free download ID9198702 What Is An Orbital In Quantum Mechanics Thinking about electrons as probabilistic matter waves using the de broglie. Shown here is the first balmer transition, in which an electron jumps from. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. In contrast to his concept of a simple circular orbit with a fixed radius, orbitals are mathematically. What Is An Orbital In Quantum Mechanics.
From www.youtube.com
Orbitals, Quantum Numbers & Electron Configuration Multiple Choice Practice Problems YouTube What Is An Orbital In Quantum Mechanics An orbital is a mathematical function that describes the matter wave of an electron — it describes the region in space where there is a high probability of finding an electron. An orbital is the quantum mechanical refinement of bohr’s orbit. The principal quantum number is one of three quantum numbers used to characterize an orbital. In hydrogen, there are. What Is An Orbital In Quantum Mechanics.
From www.youtube.com
9.6 QuantumMechanical Orbitals & Electron Configurations YouTube What Is An Orbital In Quantum Mechanics Shown here is the first balmer transition, in which an electron jumps from. The principal quantum number is one of three quantum numbers used to characterize an orbital. Thinking about electrons as probabilistic matter waves using the de broglie. Each wave function with an allowed combination of n, l, and ml values describes an atomic orbital, a particular spatial distribution.. What Is An Orbital In Quantum Mechanics.