What Does Formal Charge Mean In Chemistry . A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. This is a hypothetical measure, not a real representation of the actual. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. The sum of the formal. Formal charge (fc) is the electric charge of an atom in a molecule. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond.
from www.masterorganicchemistry.com
It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. Formal charge (fc) is the electric charge of an atom in a molecule. This is a hypothetical measure, not a real representation of the actual. The sum of the formal.
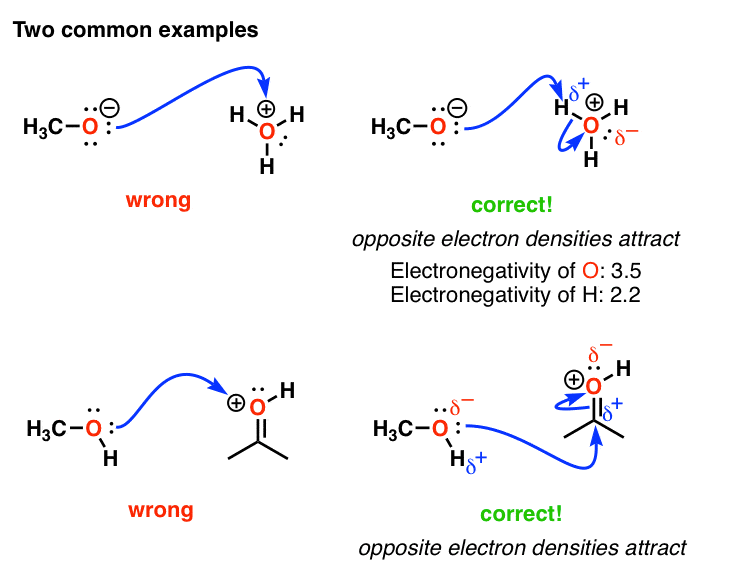
Common Mistakes In Organic Chemistry Formal Charges Can Mislead!
What Does Formal Charge Mean In Chemistry It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. This is a hypothetical measure, not a real representation of the actual. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. The sum of the formal. Formal charge (fc) is the electric charge of an atom in a molecule. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between.
From wehelpcheapessaydownload.web.fc2.com
What is the formal charge formula? What Does Formal Charge Mean In Chemistry Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. Formal charge (fc) is the electric charge of an. What Does Formal Charge Mean In Chemistry.
From chemistry291.blogspot.com
CO Lewis Structure ,Valence Electrons ,Formal Charge ,Polar or Nonpolar What Does Formal Charge Mean In Chemistry Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. This is a hypothetical measure, not a real representation of the actual. The sum of the formal. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge (fc) is. What Does Formal Charge Mean In Chemistry.
From www.youtube.com
Formal Charge YouTube What Does Formal Charge Mean In Chemistry In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. The sum of the formal. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. This. What Does Formal Charge Mean In Chemistry.
From www.organicchemistrytutor.com
Formal Charges — Organic Chemistry Tutor What Does Formal Charge Mean In Chemistry The sum of the formal. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. Formal charge is assigned to an atom in a molecule by assuming that electrons. What Does Formal Charge Mean In Chemistry.
From www.thoughtco.com
Formal Charge Definition in Chemistry What Does Formal Charge Mean In Chemistry In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. This is a hypothetical measure, not a real representation of the actual. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all. What Does Formal Charge Mean In Chemistry.
From sciencenotes.org
Element Charges Chart How to Know the Charge of an Atom What Does Formal Charge Mean In Chemistry This is a hypothetical measure, not a real representation of the actual. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. In. What Does Formal Charge Mean In Chemistry.
From www.collegesearch.in
Formal Charge Formula Definitions, Examples, Significance, Fun Facts What Does Formal Charge Mean In Chemistry It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. This is a hypothetical measure, not a real representation of the actual. A formal charge (fc) is the charge. What Does Formal Charge Mean In Chemistry.
From www.pinterest.com
Calculating NO3 Formal Charges Calculating Formal Charges for NO3 What Does Formal Charge Mean In Chemistry In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. This is a hypothetical measure, not a real representation of the actual. Formal charge (fc) is the electric charge of an atom in a molecule. A formal charge (fc) is the. What Does Formal Charge Mean In Chemistry.
From www.masterorganicchemistry.com
Common Mistakes In Organic Chemistry Formal Charges Can Mislead! What Does Formal Charge Mean In Chemistry Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. It is calculated as the number of valence electrons minus half the number of electrons shared. What Does Formal Charge Mean In Chemistry.
From sharedocnow.blogspot.com
Formula For Calculating Formal Charge sharedoc What Does Formal Charge Mean In Chemistry It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. This is a hypothetical measure, not a real representation of the actual. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. Formal charge (fc) is the electric charge. What Does Formal Charge Mean In Chemistry.
From www.masterorganicchemistry.com
Evaluating Resonance Structures With Positive Charge What Does Formal Charge Mean In Chemistry It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot. What Does Formal Charge Mean In Chemistry.
From www.youtube.com
formal chargeconcept of formal chargechemical bondingammonium Ion What Does Formal Charge Mean In Chemistry This is a hypothetical measure, not a real representation of the actual. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive. What Does Formal Charge Mean In Chemistry.
From www.studocu.com
1.1 Formal Charge Blank Practice worksheet 1 Formal Charge What is What Does Formal Charge Mean In Chemistry Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number. What Does Formal Charge Mean In Chemistry.
From ar.inspiredpencil.com
Formal Charge Dinitrogen Tetroxide What Does Formal Charge Mean In Chemistry Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. The sum of the formal. Formal charge (fc) is the electric charge of an atom in a molecule. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between.. What Does Formal Charge Mean In Chemistry.
From imagesee.biz
Font Untuk Desain Formal Charge Definition IMAGESEE What Does Formal Charge Mean In Chemistry Formal charge (fc) is the electric charge of an atom in a molecule. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. The sum of the formal. A formal charge. What Does Formal Charge Mean In Chemistry.
From www.collegesearch.in
Formal Charge Formula Definitions, Examples, Significance, Fun Facts What Does Formal Charge Mean In Chemistry Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. Formal charge (fc) is the electric charge of an atom in a molecule. This is a hypothetical measure, not a real representation of the actual. Formal charge is assigned to an atom in a molecule by assuming that. What Does Formal Charge Mean In Chemistry.
From www.youtube.com
AP Chemistry Unit 32 Formal charges & Hybridization YouTube What Does Formal Charge Mean In Chemistry It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. Formal charge is a charge assigned to an atom under the assumption that all electrons in. What Does Formal Charge Mean In Chemistry.
From sciencenotes.org
Periodic Table With Charges What Does Formal Charge Mean In Chemistry Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. The sum of the formal. Formal charge (fc) is the electric charge of an atom in a molecule.. What Does Formal Charge Mean In Chemistry.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry What Does Formal Charge Mean In Chemistry This is a hypothetical measure, not a real representation of the actual. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. Formal charge is a charge assigned to. What Does Formal Charge Mean In Chemistry.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry What Does Formal Charge Mean In Chemistry Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. The sum of the formal. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. In the previous post, we talked about the covalent bond. What Does Formal Charge Mean In Chemistry.
From solvedlib.com
Problem 6Determine the formal charge on the labeled … SolvedLib What Does Formal Charge Mean In Chemistry The sum of the formal. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. In the previous post, we talked about the covalent bond and mentioned that. What Does Formal Charge Mean In Chemistry.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry What Does Formal Charge Mean In Chemistry It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. The sum of the formal. Formal charge is a charge assigned to. What Does Formal Charge Mean In Chemistry.
From www.masterorganicchemistry.com
7 Factors That Stabilize Positive Charge in Organic Chemistry Master What Does Formal Charge Mean In Chemistry Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. Formal charge (fc) is the electric charge of an atom in a molecule.. What Does Formal Charge Mean In Chemistry.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry What Does Formal Charge Mean In Chemistry Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. The sum of the formal. Formal charge (fc) is the electric charge of an atom in a molecule.. What Does Formal Charge Mean In Chemistry.
From www.organicchemistrytutor.com
Formal Charges — Organic Chemistry Tutor What Does Formal Charge Mean In Chemistry Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. The sum of the formal. Formal charge is a charge assigned to an atom under the assumption that all. What Does Formal Charge Mean In Chemistry.
From www.expii.com
Formal Charge — Overview & Calculation Expii What Does Formal Charge Mean In Chemistry Formal charge (fc) is the electric charge of an atom in a molecule. Formal charge are a way of keeping track of where the electrons an atom donates to a lewis dot structure are placed. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have. What Does Formal Charge Mean In Chemistry.
From chemistry.stackexchange.com
organic chemistry How to calculate formal charge? Chemistry Stack What Does Formal Charge Mean In Chemistry In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. The sum of the formal. Formal charge is assigned to an atom. What Does Formal Charge Mean In Chemistry.
From www.masterorganicchemistry.com
How To Calculate Formal Charge What Does Formal Charge Mean In Chemistry This is a hypothetical measure, not a real representation of the actual. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have either a positive or. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond.. What Does Formal Charge Mean In Chemistry.
From www.chemistrysteps.com
Formal Charges in Organic Chemistry Chemistry Steps What Does Formal Charge Mean In Chemistry It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. Formal charge are a way of keeping track of where the electrons an atom donates to. What Does Formal Charge Mean In Chemistry.
From www.slideserve.com
PPT FORMAL CHARGE PowerPoint Presentation, free download ID4196511 What Does Formal Charge Mean In Chemistry A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. This is a hypothetical measure, not a real representation of the actual. Formal. What Does Formal Charge Mean In Chemistry.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry What Does Formal Charge Mean In Chemistry Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. Formal charge (fc) is the electric charge of an atom in a molecule. In the previous post, we talked about the covalent bond and mentioned that sometimes, depending on the number of bonds, the given atom might have. What Does Formal Charge Mean In Chemistry.
From www.slideserve.com
PPT Formal Charge PowerPoint Presentation, free download ID338614 What Does Formal Charge Mean In Chemistry Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. The sum of the formal. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. Formal charge are a way of keeping track. What Does Formal Charge Mean In Chemistry.
From study.com
Formal Charge Definition, Formula & Calculation Methods Lesson What Does Formal Charge Mean In Chemistry Formal charge (fc) is the electric charge of an atom in a molecule. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge is a charge assigned to an atom under the assumption that all electrons in bonds are shared equally. Formal charge is assigned to an atom in. What Does Formal Charge Mean In Chemistry.
From www.youtube.com
How to Calculate the Formal Charges for PO4 3 (Phosphate ion) YouTube What Does Formal Charge Mean In Chemistry Formal charge (fc) is the electric charge of an atom in a molecule. A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. It is calculated as the number of valence electrons minus half the number of electrons shared in a bond. Formal charge is. What Does Formal Charge Mean In Chemistry.
From www.youtube.com
DIFFERENT FORMULA FOR FORMAL CHARGE//CHEMICAL BONDING//FOR FOUNDATION What Does Formal Charge Mean In Chemistry A formal charge (fc) is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between. Formal charge is assigned to an atom in a molecule by assuming that electrons in all chemical bonds are shared equally between. Formal charge is a charge assigned to an atom under the assumption that. What Does Formal Charge Mean In Chemistry.