Dilution Nomenclature . The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. A concentrated solution contains a. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution by changing the. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of.
from www.numerade.com
Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how to dilute and concentrate solutions. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. Often, a worker will need to change the concentration of a solution by changing the. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. Concentration is the removal of. A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application.
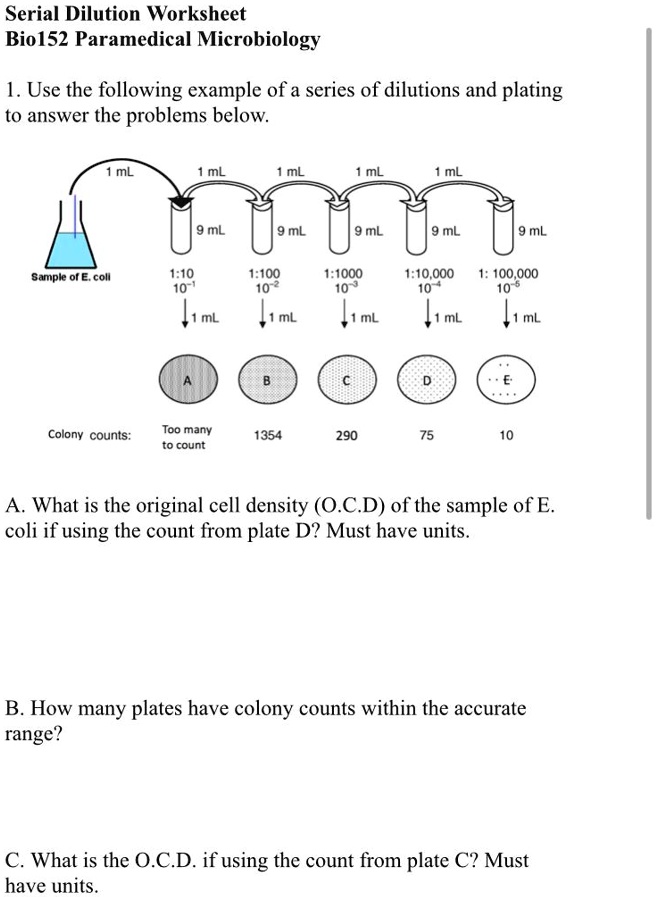
SOLVED Serial Dilution Worksheet Bio152 Paramedical Microbiology Use
Dilution Nomenclature Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the. Concentration is the removal of. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. A concentrated solution contains a. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how to dilute and concentrate solutions. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.
From stock.adobe.com
The tenfold serial dilution of pathogen suspension in solution sample Dilution Nomenclature Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. Often,. Dilution Nomenclature.
From www.freepik.com
Premium Vector Serial Dilutions science vector illustration infographic Dilution Nomenclature Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Dilution Nomenclature.
From www.chegg.com
his the flow diagram in EX 18 that you would have Dilution Nomenclature State whether the concentration of a solution is directly or indirectly proportional to its volume. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Concentration is the removal of. A dilute solution is one in which there is a relatively small amount of solute dissolved in. Dilution Nomenclature.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Dilution Nomenclature State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. A concentrated solution contains a. The goal of dilution. Dilution Nomenclature.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Dilution Nomenclature A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution by changing the. Concentration is the removal of. Learn how to dilute and. Dilution Nomenclature.
From moniqueaafiyah.blogspot.com
Share dilution calculator MoniqueAafiyah Dilution Nomenclature Learn how to dilute and concentrate solutions. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Katherine dorfman, umass biology department, 2019 it is often very important. Dilution Nomenclature.
From www.etsy.com
Dilution Guide Serial & Normal Clinical Laboratory Dilutions Mlt/mls/mt Dilution Nomenclature State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A concentrated solution contains a. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. Often, a. Dilution Nomenclature.
From www.youtube.com
Serial Dilution Calculations YouTube Dilution Nomenclature State whether the concentration of a solution is directly or indirectly proportional to its volume. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Often, a worker will need to change the concentration of a solution. Dilution Nomenclature.
From www.biorender.com
Serial Dilution Procedure BioRender Science Templates Dilution Nomenclature State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. Learn how to dilute and concentrate solutions. A concentrated solution contains. Dilution Nomenclature.
From www.chegg.com
Solved Serial dilution is a common technique used in Dilution Nomenclature Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Learn how to dilute and concentrate solutions. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. A dilute solution is one in which there is a relatively small amount of. Dilution Nomenclature.
From www.studocu.com
Tube dilution method best Tube dilutionn method The method involves Dilution Nomenclature Often, a worker will need to change the concentration of a solution by changing the. Concentration is the removal of. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the. Dilution Nomenclature.
From www.doubtnut.com
Use of dilution formula (M(1)V(1) = M(2) V(2)) Dilution Nomenclature The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilutions can be performed in. Dilution Nomenclature.
From www.youtube.com
Serial Dilutions YouTube Dilution Nomenclature Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. State whether the concentration of a solution is directly or indirectly proportional to its volume. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. A concentrated solution contains a. Concentration is the removal. Dilution Nomenclature.
From fabrikbrands.com
What Is Brand Dilution? Brand Dilution Definition With Examples Dilution Nomenclature The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Often, a worker will need to change the concentration of a solution by changing the. State whether the concentration of a solution is directly or indirectly proportional to its volume. A concentrated solution contains a. A dilute. Dilution Nomenclature.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Nomenclature Learn how to dilute and concentrate solutions. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution by changing the. State whether the concentration of a solution is directly or indirectly proportional to its volume. The goal of dilution. Dilution Nomenclature.
From www.chegg.com
Solved Dilution diagrams can be very helpful in organizing Dilution Nomenclature Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. Learn how to dilute and concentrate solutions. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. A dilute solution is one in which there is a relatively. Dilution Nomenclature.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Nomenclature The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one in which there is a relatively small amount of solute dissolved. Dilution Nomenclature.
From microbialnotes.com
Serial dilution how do we prepare it? Dilution Nomenclature State whether the concentration of a solution is directly or indirectly proportional to its volume. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. A concentrated solution. Dilution Nomenclature.
From www.youtube.com
How to Perform Serial Dilution for Bacterial Growth Measurement Step Dilution Nomenclature State whether the concentration of a solution is directly or indirectly proportional to its volume. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Katherine dorfman, umass biology department, 2019. Dilution Nomenclature.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Nomenclature Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Dilution. Dilution Nomenclature.
From chem370.github.io
Lab 1 Prelab CHEM 370 Dilution Nomenclature Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilutions can be performed in the. Dilution Nomenclature.
From ar.inspiredpencil.com
Serial Dilution Diagram Dilution Nomenclature Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. A concentrated solution contains a. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. Concentration is the removal of. The goal of dilution is to create a solution with a lower concentration that. Dilution Nomenclature.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Nomenclature Learn how to dilute and concentrate solutions. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. State whether the concentration of a solution is directly or indirectly. Dilution Nomenclature.
From fr.thptnganamst.edu.vn
Découvrir 184+ imagen dilution formule fr.thptnganamst.edu.vn Dilution Nomenclature Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. A concentrated solution contains a. Often, a worker. Dilution Nomenclature.
From www.hanlin.com
IB DP Biology HL复习笔记2.4.3 Skills Enzyme Experiments翰林国际教育 Dilution Nomenclature Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. State whether the. Dilution Nomenclature.
From www.learnsci.com
LearnSci Smart Worksheet Serial Dilutions Dilution Nomenclature Concentration is the removal of. Learn how to dilute and concentrate solutions. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of. Dilution Nomenclature.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Nomenclature Often, a worker will need to change the concentration of a solution by changing the. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. Dilution is. Dilution Nomenclature.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query Dilution Nomenclature Concentration is the removal of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A concentrated solution contains a. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy.. Dilution Nomenclature.
From www.researchgate.net
Procedures of serial dilution preparation Download Scientific Diagram Dilution Nomenclature A concentrated solution contains a. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. Concentration is the removal of. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Often, a worker will need to change the concentration of a solution. Dilution Nomenclature.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Nomenclature Katherine dorfman, umass biology department, 2019 it is often very important to know the precise concentration of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Often, a worker will need to change the concentration of a solution by changing the. A concentrated solution contains a. Concentration is the removal of. The goal of. Dilution Nomenclature.
From www.numerade.com
SOLVED Serial Dilution Worksheet Bio152 Paramedical Microbiology Use Dilution Nomenclature Learn how to dilute and concentrate solutions. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of. A dilute solution is one in which there is a relatively small amount of. Dilution Nomenclature.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Nomenclature Concentration is the removal of. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Learn how to dilute and concentrate solutions. A concentrated solution contains a. Katherine dorfman, umass biology department, 2019 it is often. Dilution Nomenclature.
From thestudentnotes.com
Serial Dilution Definition, Formula, Calculator, Procedure, Uses The Dilution Nomenclature Learn how to dilute and concentrate solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Dilutions can be. Dilution Nomenclature.
From memoyellow855.weebly.com
Bacterial Serial Dilution Method memoyellow Dilution Nomenclature A concentrated solution contains a. Dilutions can be performed in the laboratory with various tools, depending on the volumes required and the desired accuracy. State whether the concentration of a solution is directly or indirectly proportional to its volume. A dilute solution is one in which there is a relatively small amount of solute dissolved in the solution. Learn how. Dilution Nomenclature.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution Nomenclature Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. A concentrated solution contains a. The goal of dilution is to create a solution with a lower concentration that is suitable for a particular experiment, analysis, or application. Learn. Dilution Nomenclature.