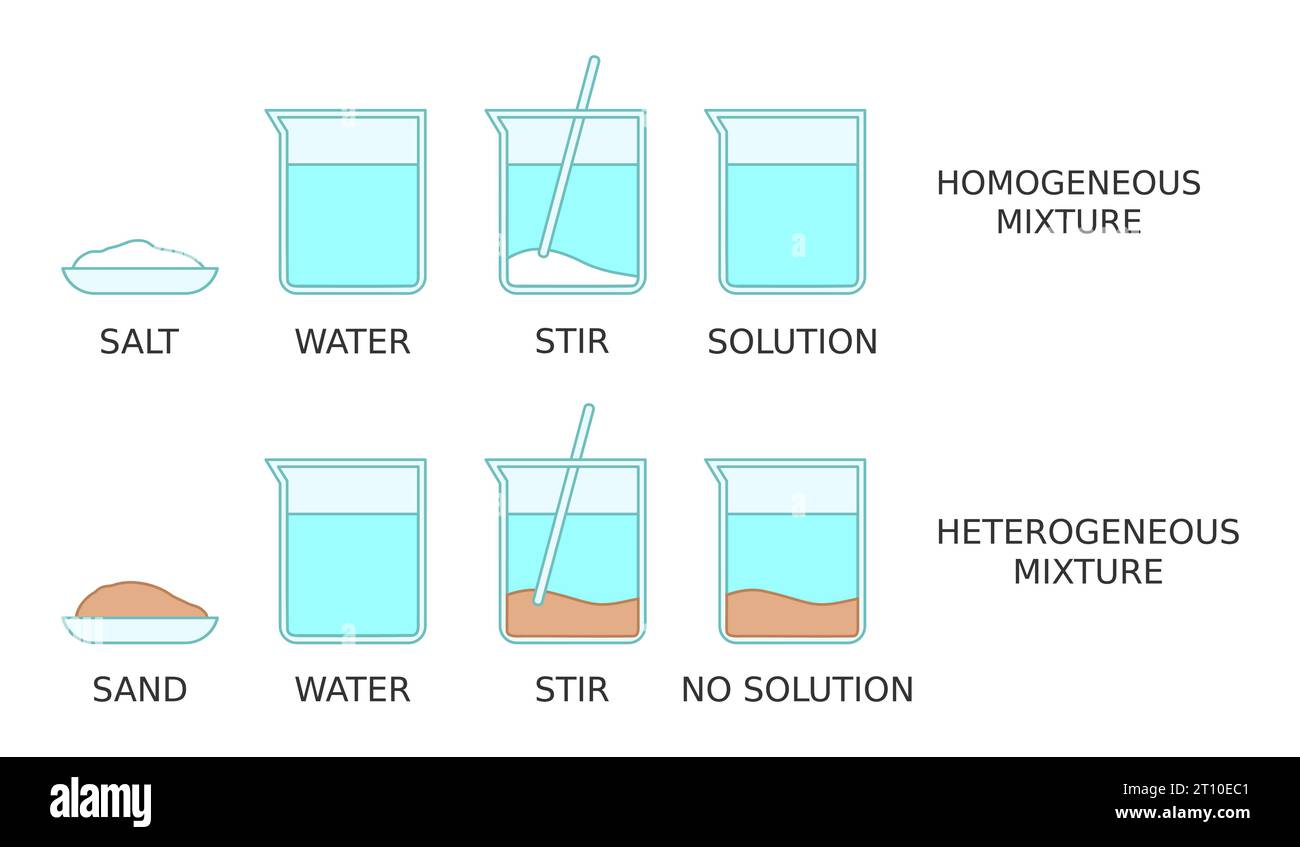
What Substances Cannot Dissolve In Water . When put into polar environments, such as water, nonpolar. They are described as hydrophobic, or water fearing. When a substance dissolves in water, you can’t. Heating, stirring and using fine. Sand, fats, wood, metals, and. Insoluble generally means that a substance does not dissolve in water. It consists of two hydrogens connected to. Water is called a universal solvent. Nonpolar molecules do not dissolve easily in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. We’re going to investigate which solids dissolve in water. If it cannot dissolve, it is described as insoluble. Nonelectrolytes are substances that do not produce ions when. It provides a basic support for the life of all living organisms. For example, salt and sugar are both soluble in water.
from www.alamy.com
Substances that dissolve in water to yield ions are called electrolytes. Heating, stirring and using fine. When a substance dissolves in water, you can’t. Insoluble generally means that a substance does not dissolve in water. For example, salt and sugar are both soluble in water. They are described as hydrophobic, or water fearing. When put into polar environments, such as water, nonpolar. We’re going to investigate which solids dissolve in water. It consists of two hydrogens connected to. Nonelectrolytes are substances that do not produce ions when.
Solution science experiment. Solubility of salt and sand in water
What Substances Cannot Dissolve In Water We’re going to investigate which solids dissolve in water. If it cannot dissolve, it is described as insoluble. It consists of two hydrogens connected to. Sand, fats, wood, metals, and. Water is called a universal solvent. Nonelectrolytes are substances that do not produce ions when. They are described as hydrophobic, or water fearing. When a substance dissolves in water, you can’t. When put into polar environments, such as water, nonpolar. It provides a basic support for the life of all living organisms. Heating, stirring and using fine. Nonpolar molecules do not dissolve easily in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Insoluble generally means that a substance does not dissolve in water. For example, salt and sugar are both soluble in water. Substances that dissolve in water to yield ions are called electrolytes.
From www.nagwa.com
Question Video Using the Water Solubility Rules to Determine Which What Substances Cannot Dissolve In Water Sand, fats, wood, metals, and. If it cannot dissolve, it is described as insoluble. Water is called a universal solvent. Insoluble generally means that a substance does not dissolve in water. They are described as hydrophobic, or water fearing. Heating, stirring and using fine. Nonpolar molecules do not dissolve easily in water. Water, which not only dissolves many compounds but. What Substances Cannot Dissolve In Water.
From www.youtube.com
Why oil does not dissolve in water Polarity of water YouTube What Substances Cannot Dissolve In Water They are described as hydrophobic, or water fearing. It provides a basic support for the life of all living organisms. Heating, stirring and using fine. We’re going to investigate which solids dissolve in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. It consists of two hydrogens connected to. Nonelectrolytes are. What Substances Cannot Dissolve In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Substances Cannot Dissolve In Water Nonpolar molecules do not dissolve easily in water. When a substance dissolves in water, you can’t. Insoluble generally means that a substance does not dissolve in water. Water is called a universal solvent. If it cannot dissolve, it is described as insoluble. Nonelectrolytes are substances that do not produce ions when. We’re going to investigate which solids dissolve in water.. What Substances Cannot Dissolve In Water.
From byjus.com
Which among the following things do not dissolve in water? What Substances Cannot Dissolve In Water It consists of two hydrogens connected to. When a substance dissolves in water, you can’t. It provides a basic support for the life of all living organisms. Sand, fats, wood, metals, and. When put into polar environments, such as water, nonpolar. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Nonelectrolytes are. What Substances Cannot Dissolve In Water.
From www.slideserve.com
PPT Chemistry of Life PowerPoint Presentation ID5875746 What Substances Cannot Dissolve In Water Heating, stirring and using fine. It consists of two hydrogens connected to. When a substance dissolves in water, you can’t. Nonelectrolytes are substances that do not produce ions when. We’re going to investigate which solids dissolve in water. They are described as hydrophobic, or water fearing. Water is called a universal solvent. Sand, fats, wood, metals, and. When put into. What Substances Cannot Dissolve In Water.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids What Substances Cannot Dissolve In Water When put into polar environments, such as water, nonpolar. Nonelectrolytes are substances that do not produce ions when. It provides a basic support for the life of all living organisms. Heating, stirring and using fine. When a substance dissolves in water, you can’t. They are described as hydrophobic, or water fearing. Insoluble generally means that a substance does not dissolve. What Substances Cannot Dissolve In Water.
From www.dreamstime.com
How Does Sodium Chloride NaCl Dissolve in Water Stock Vector What Substances Cannot Dissolve In Water Insoluble generally means that a substance does not dissolve in water. Nonpolar molecules do not dissolve easily in water. Water is called a universal solvent. Sand, fats, wood, metals, and. It consists of two hydrogens connected to. We’re going to investigate which solids dissolve in water. Substances that dissolve in water to yield ions are called electrolytes. When put into. What Substances Cannot Dissolve In Water.
From littlebinsforlittlehands.com
What Dissolves In Water Experiment Little Bins for Little Hands What Substances Cannot Dissolve In Water Sand, fats, wood, metals, and. When put into polar environments, such as water, nonpolar. Heating, stirring and using fine. Water is called a universal solvent. It provides a basic support for the life of all living organisms. When a substance dissolves in water, you can’t. They are described as hydrophobic, or water fearing. Nonpolar molecules do not dissolve easily in. What Substances Cannot Dissolve In Water.
From slideplayer.com
Bio Today Properties of Water Notes & Lab ppt download What Substances Cannot Dissolve In Water Nonpolar molecules do not dissolve easily in water. It provides a basic support for the life of all living organisms. If it cannot dissolve, it is described as insoluble. When a substance dissolves in water, you can’t. Water is called a universal solvent. Insoluble generally means that a substance does not dissolve in water. We’re going to investigate which solids. What Substances Cannot Dissolve In Water.
From teachbesideme.com
Dissolving Science Experiment What Dissolves in Water? Teach Beside Me What Substances Cannot Dissolve In Water Nonpolar molecules do not dissolve easily in water. They are described as hydrophobic, or water fearing. If it cannot dissolve, it is described as insoluble. It consists of two hydrogens connected to. Nonelectrolytes are substances that do not produce ions when. It provides a basic support for the life of all living organisms. When a substance dissolves in water, you. What Substances Cannot Dissolve In Water.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize What Substances Cannot Dissolve In Water Water is called a universal solvent. Sand, fats, wood, metals, and. When a substance dissolves in water, you can’t. They are described as hydrophobic, or water fearing. Nonelectrolytes are substances that do not produce ions when. It consists of two hydrogens connected to. We’re going to investigate which solids dissolve in water. Nonpolar molecules do not dissolve easily in water.. What Substances Cannot Dissolve In Water.
From www.youtube.com
Why Does This Powder Only Dissolve In Cold Water? YouTube What Substances Cannot Dissolve In Water It consists of two hydrogens connected to. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Sand, fats, wood, metals, and. They are described as hydrophobic, or water fearing. Heating, stirring and using fine. For example, salt and sugar are both soluble in water. Substances that dissolve in water to yield ions. What Substances Cannot Dissolve In Water.
From ar.inspiredpencil.com
Dissolving In Water What Substances Cannot Dissolve In Water We’re going to investigate which solids dissolve in water. Heating, stirring and using fine. Nonpolar molecules do not dissolve easily in water. Water is called a universal solvent. For example, salt and sugar are both soluble in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Substances that dissolve in water. What Substances Cannot Dissolve In Water.
From www.youtube.com
DYK Water can dissolve more substances than any other liquid because What Substances Cannot Dissolve In Water Water is called a universal solvent. We’re going to investigate which solids dissolve in water. Sand, fats, wood, metals, and. For example, salt and sugar are both soluble in water. Nonpolar molecules do not dissolve easily in water. It provides a basic support for the life of all living organisms. Insoluble generally means that a substance does not dissolve in. What Substances Cannot Dissolve In Water.
From slideplayer.com
Solutions. ppt download What Substances Cannot Dissolve In Water For example, salt and sugar are both soluble in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Nonelectrolytes are substances that do not produce ions when. When put into polar environments, such as water, nonpolar. It provides a basic support for the life of all living organisms. Water is called. What Substances Cannot Dissolve In Water.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3333021 What Substances Cannot Dissolve In Water It provides a basic support for the life of all living organisms. Insoluble generally means that a substance does not dissolve in water. They are described as hydrophobic, or water fearing. Water is called a universal solvent. We’re going to investigate which solids dissolve in water. When put into polar environments, such as water, nonpolar. It consists of two hydrogens. What Substances Cannot Dissolve In Water.
From solutionpharmacy.in
Factors Influencing Solubility Solution Parmacy What Substances Cannot Dissolve In Water It consists of two hydrogens connected to. Insoluble generally means that a substance does not dissolve in water. Heating, stirring and using fine. For example, salt and sugar are both soluble in water. Water is called a universal solvent. Sand, fats, wood, metals, and. Substances that dissolve in water to yield ions are called electrolytes. Water, which not only dissolves. What Substances Cannot Dissolve In Water.
From www.youtube.com
Which substances dissolve in water? YouTube What Substances Cannot Dissolve In Water Heating, stirring and using fine. For example, salt and sugar are both soluble in water. We’re going to investigate which solids dissolve in water. They are described as hydrophobic, or water fearing. When put into polar environments, such as water, nonpolar. It consists of two hydrogens connected to. Insoluble generally means that a substance does not dissolve in water. It. What Substances Cannot Dissolve In Water.
From www.slideserve.com
PPT Unit 6 TOXINS Solutions & PowerPoint Presentation ID What Substances Cannot Dissolve In Water They are described as hydrophobic, or water fearing. Sand, fats, wood, metals, and. When a substance dissolves in water, you can’t. Substances that dissolve in water to yield ions are called electrolytes. Water is called a universal solvent. Heating, stirring and using fine. Nonpolar molecules do not dissolve easily in water. It consists of two hydrogens connected to. We’re going. What Substances Cannot Dissolve In Water.
From www.cbsetuts.com
Which of the following substances are insoluble in water? CBSE Tuts What Substances Cannot Dissolve In Water We’re going to investigate which solids dissolve in water. Insoluble generally means that a substance does not dissolve in water. If it cannot dissolve, it is described as insoluble. When a substance dissolves in water, you can’t. Substances that dissolve in water to yield ions are called electrolytes. For example, salt and sugar are both soluble in water. Sand, fats,. What Substances Cannot Dissolve In Water.
From www.dreamstime.com
Dissolving Science Experiment with Sand and Water Stock Vector What Substances Cannot Dissolve In Water Heating, stirring and using fine. Substances that dissolve in water to yield ions are called electrolytes. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Nonelectrolytes are substances that do not produce ions when. It consists of two hydrogens connected to. It provides a basic support for the life of all living. What Substances Cannot Dissolve In Water.
From www.twinkl.fr
What is Dissolving? Answered Twinkl Teaching Wiki What Substances Cannot Dissolve In Water For example, salt and sugar are both soluble in water. It consists of two hydrogens connected to. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Nonelectrolytes are substances that do not produce ions when. Nonpolar molecules do not dissolve easily in water. When a substance dissolves in water, you can’t. It. What Substances Cannot Dissolve In Water.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? What Substances Cannot Dissolve In Water When put into polar environments, such as water, nonpolar. Heating, stirring and using fine. Nonpolar molecules do not dissolve easily in water. Insoluble generally means that a substance does not dissolve in water. We’re going to investigate which solids dissolve in water. If it cannot dissolve, it is described as insoluble. It provides a basic support for the life of. What Substances Cannot Dissolve In Water.
From www.alamy.com
Solution science experiment. Solubility of salt and sand in water What Substances Cannot Dissolve In Water For example, salt and sugar are both soluble in water. Nonelectrolytes are substances that do not produce ions when. Water is called a universal solvent. When put into polar environments, such as water, nonpolar. Insoluble generally means that a substance does not dissolve in water. They are described as hydrophobic, or water fearing. Nonpolar molecules do not dissolve easily in. What Substances Cannot Dissolve In Water.
From vestals21stcenturyclassroom.com
What Dissolves in Water Study Guide What Substances Cannot Dissolve In Water It consists of two hydrogens connected to. Water is called a universal solvent. Sand, fats, wood, metals, and. Heating, stirring and using fine. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Insoluble generally means that a substance does not dissolve in water. If it cannot dissolve, it is described as insoluble.. What Substances Cannot Dissolve In Water.
From teachbesideme.com
Dissolving Science Experiment What Dissolves in Water? Teach Beside Me What Substances Cannot Dissolve In Water When a substance dissolves in water, you can’t. Heating, stirring and using fine. Water is called a universal solvent. Nonelectrolytes are substances that do not produce ions when. Nonpolar molecules do not dissolve easily in water. If it cannot dissolve, it is described as insoluble. We’re going to investigate which solids dissolve in water. Water, which not only dissolves many. What Substances Cannot Dissolve In Water.
From sciencenotes.org
Why Is Water Called the Universal Solvent? What Substances Cannot Dissolve In Water We’re going to investigate which solids dissolve in water. It consists of two hydrogens connected to. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Water is called a universal solvent. Nonpolar molecules do not dissolve easily in water. If it cannot dissolve, it is described as insoluble. They are described as. What Substances Cannot Dissolve In Water.
From www.slideserve.com
PPT Chapter 2 Water PowerPoint Presentation, free download ID262198 What Substances Cannot Dissolve In Water It provides a basic support for the life of all living organisms. For example, salt and sugar are both soluble in water. Nonelectrolytes are substances that do not produce ions when. Insoluble generally means that a substance does not dissolve in water. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. Heating,. What Substances Cannot Dissolve In Water.
From brainly.in
the substance that will not dissolve in water Brainly.in What Substances Cannot Dissolve In Water Heating, stirring and using fine. Nonpolar molecules do not dissolve easily in water. They are described as hydrophobic, or water fearing. Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. When put into polar environments, such as water, nonpolar. For example, salt and sugar are both soluble in water. When a substance. What Substances Cannot Dissolve In Water.
From slideplayer.com
Properties of Water. ppt download What Substances Cannot Dissolve In Water Sand, fats, wood, metals, and. They are described as hydrophobic, or water fearing. Nonelectrolytes are substances that do not produce ions when. We’re going to investigate which solids dissolve in water. It provides a basic support for the life of all living organisms. When put into polar environments, such as water, nonpolar. It consists of two hydrogens connected to. For. What Substances Cannot Dissolve In Water.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID What Substances Cannot Dissolve In Water Water, which not only dissolves many compounds but also dissolves more substances than any other liquid, is. We’re going to investigate which solids dissolve in water. Sand, fats, wood, metals, and. For example, salt and sugar are both soluble in water. It provides a basic support for the life of all living organisms. When put into polar environments, such as. What Substances Cannot Dissolve In Water.
From socratic.org
What determines whether a solid is soluble in water? Socratic What Substances Cannot Dissolve In Water They are described as hydrophobic, or water fearing. If it cannot dissolve, it is described as insoluble. Water is called a universal solvent. It consists of two hydrogens connected to. When put into polar environments, such as water, nonpolar. Sand, fats, wood, metals, and. Nonelectrolytes are substances that do not produce ions when. We’re going to investigate which solids dissolve. What Substances Cannot Dissolve In Water.
From slideplayer.com
H2O Just Add Water. ppt download What Substances Cannot Dissolve In Water If it cannot dissolve, it is described as insoluble. When a substance dissolves in water, you can’t. For example, salt and sugar are both soluble in water. We’re going to investigate which solids dissolve in water. Heating, stirring and using fine. It provides a basic support for the life of all living organisms. When put into polar environments, such as. What Substances Cannot Dissolve In Water.
From www.slideserve.com
PPT LIFE DEPENDS ON THE UNIQUE PROPERITIES OF WATER PowerPoint What Substances Cannot Dissolve In Water Insoluble generally means that a substance does not dissolve in water. When a substance dissolves in water, you can’t. It provides a basic support for the life of all living organisms. It consists of two hydrogens connected to. When put into polar environments, such as water, nonpolar. Water is called a universal solvent. Nonelectrolytes are substances that do not produce. What Substances Cannot Dissolve In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation ID2276550 What Substances Cannot Dissolve In Water Insoluble generally means that a substance does not dissolve in water. Substances that dissolve in water to yield ions are called electrolytes. When put into polar environments, such as water, nonpolar. When a substance dissolves in water, you can’t. Sand, fats, wood, metals, and. Heating, stirring and using fine. It provides a basic support for the life of all living. What Substances Cannot Dissolve In Water.