Table Salt Reaction With H2So4 . the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. why some acid/alkali can react with some salt, forming another acid/alkali? some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. They react with each other to make water and an ionic compound called a salt. neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. acids and bases have another property: the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic.
from www.slideserve.com
the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. They react with each other to make water and an ionic compound called a salt. neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. acids and bases have another property: some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. why some acid/alkali can react with some salt, forming another acid/alkali?
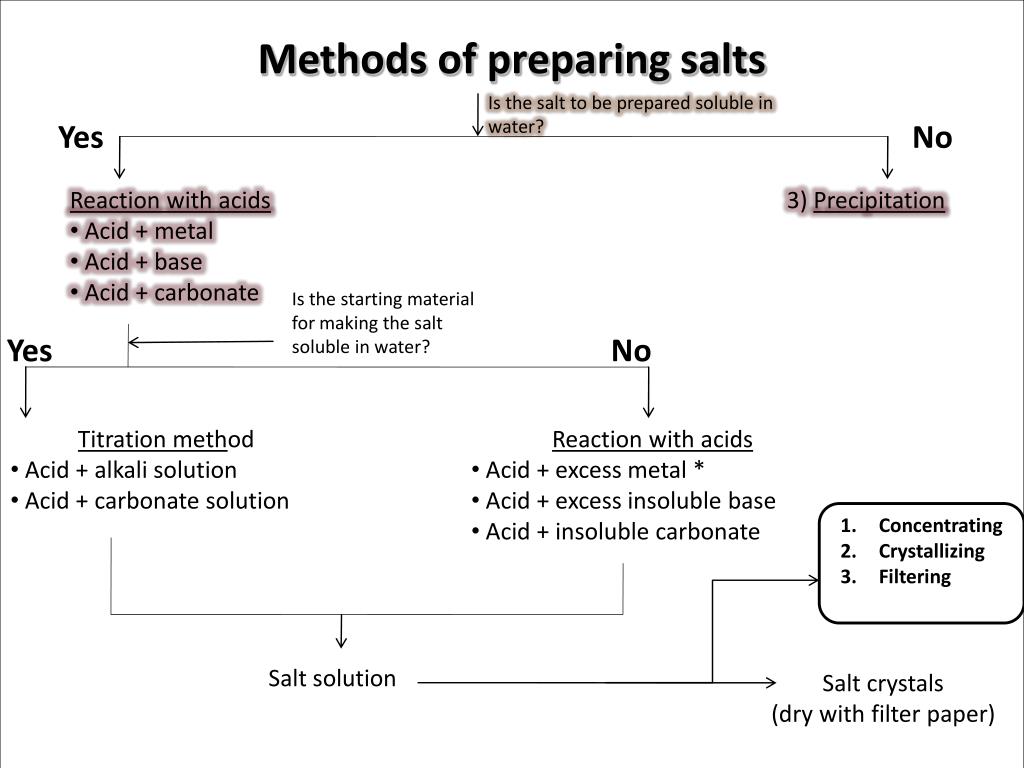
PPT Preparation of Salts PowerPoint Presentation, free download ID
Table Salt Reaction With H2So4 neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. why some acid/alkali can react with some salt, forming another acid/alkali? neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. acids and bases have another property: some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. They react with each other to make water and an ionic compound called a salt.
From byjus.com
What are the products obtained from the reaction of sodium chloride and Table Salt Reaction With H2So4 neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. acids and bases have another property: why some acid/alkali can react with some salt, forming another acid/alkali? some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. salt hydrolysis. Table Salt Reaction With H2So4.
From www.chegg.com
Solved What is the effect of H2SO4 on table salt? Select all Table Salt Reaction With H2So4 neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. acids and bases have another property: They react with each other to make water and an ionic compound called a salt. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. the reaction of. Table Salt Reaction With H2So4.
From www.youtube.com
Barium Chloride and Sulfuric Acid = ?? YouTube Table Salt Reaction With H2So4 acids and bases have another property: neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. some people use it to remove stumps, etc., because it reacts pretty. Table Salt Reaction With H2So4.
From www.youtube.com
Identify hydrochloric acid and sulfuric acid solutions HCl and H2SO4 Table Salt Reaction With H2So4 neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. acids and bases have another property: the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. They react with each other to make water and an ionic compound called a salt. some people use. Table Salt Reaction With H2So4.
From clarktivesoto.blogspot.com
Potassium Hydroxide and Sulphuric Acid Table Salt Reaction With H2So4 the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. They react with each other to make water and an ionic compound called a salt. why some acid/alkali can react with some salt, forming another acid/alkali? salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either. Table Salt Reaction With H2So4.
From onlinecalculator.guru
Salt Analysis Formulas Complete List Formulae Sheet for Salt Analysis Table Salt Reaction With H2So4 neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. They react with each other to make water and an ionic compound called a salt. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic.. Table Salt Reaction With H2So4.
From www.youtube.com
Equation for (NH4)2SO4 + H2O (Ammonium sulfate + Water) YouTube Table Salt Reaction With H2So4 salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. They react with each other to make water and an ionic compound called a salt. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. acids and bases have another property: . Table Salt Reaction With H2So4.
From www.researchgate.net
[3] The heat of H2SO4 dissolving in the water. Download Table Table Salt Reaction With H2So4 acids and bases have another property: salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. They react with each other to make water. Table Salt Reaction With H2So4.
From www.studypool.com
SOLUTION Produce two different metal salts nahso4 and na2so4 through Table Salt Reaction With H2So4 acids and bases have another property: They react with each other to make water and an ionic compound called a salt. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. . Table Salt Reaction With H2So4.
From www.masterorganicchemistry.com
Nitration and Sulfonation Reactions In Electrophilic Aromatic Substitution Table Salt Reaction With H2So4 acids and bases have another property: the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. why some acid/alkali can react with some salt, forming another acid/alkali? They react with each other to make water and an ionic compound called a salt. salt hydrolysis is a reaction in. Table Salt Reaction With H2So4.
From learnah.org
4 Reaction of halide salts with concentrated sulfuric acid Table Salt Reaction With H2So4 the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. why some acid/alkali can react with some salt, forming another acid/alkali? neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. acids and bases have another property: They react with each other to make. Table Salt Reaction With H2So4.
From www.toppr.com
On treatment with dilute H2SO4 , a salt gives a gas which turns lime Table Salt Reaction With H2So4 They react with each other to make water and an ionic compound called a salt. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. salt hydrolysis is a reaction in which one of the ions from a salt. Table Salt Reaction With H2So4.
From brainly.in
calculate the equivalent mass of the H2So4 Brainly.in Table Salt Reaction With H2So4 why some acid/alkali can react with some salt, forming another acid/alkali? the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. neutralization reactions are one type of chemical reaction that proceeds. Table Salt Reaction With H2So4.
From quizdbmonarchist.z21.web.core.windows.net
What Salt Is Formed From Sulfuric Acid Table Salt Reaction With H2So4 the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. acids and bases have another property: why some acid/alkali can react with some salt, forming another acid/alkali? some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. salt hydrolysis is a. Table Salt Reaction With H2So4.
From www.toppr.com
The diagram represents the preparation of sodium sulphate salt from Table Salt Reaction With H2So4 the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. acids and bases have another property: the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. why some acid/alkali can react with some salt, forming another acid/alkali? They react with each other to make water and. Table Salt Reaction With H2So4.
From www.youtube.com
How to Write the Net Ionic Equation for NH3 + H2SO4 = (NH4)2SO4 YouTube Table Salt Reaction With H2So4 salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. acids and bases have another property: the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should. Table Salt Reaction With H2So4.
From www.slideserve.com
PPT ACIDS, BASES AND SALTS PowerPoint Presentation, free download Table Salt Reaction With H2So4 the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either. Table Salt Reaction With H2So4.
From www.coursehero.com
[Solved] . 2. Provide the major product for the following reactions Table Salt Reaction With H2So4 They react with each other to make water and an ionic compound called a salt. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. why some acid/alkali can react with some. Table Salt Reaction With H2So4.
From www.youtube.com
Sodium Hydroxide + Sulfuric Acid Acid Base Neutralization Reaction Table Salt Reaction With H2So4 the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. They react with each other to make water and an ionic compound called a salt. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. acids and bases have another property: salt hydrolysis is a reaction. Table Salt Reaction With H2So4.
From www.toppr.com
Fill the missing data in the following table.Name of the Table Salt Reaction With H2So4 the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. acids and bases have another property: why some acid/alkali can react with some salt, forming another acid/alkali? some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. neutralization reactions are one. Table Salt Reaction With H2So4.
From www.toppr.com
Fill the missing data in the following table.Name of the Table Salt Reaction With H2So4 some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. They react with each other to make water and an ionic compound called a salt. the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. acids and bases have another property: the. Table Salt Reaction With H2So4.
From www.youtube.com
How to Balance Ca(OH)2 + H2SO4 = CaSO4 + H2O (Calcium Hydroxide plus Table Salt Reaction With H2So4 the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. They react with each other to make water and an ionic compound called a salt. why some acid/alkali can react with some salt, forming another acid/alkali? acids and bases have another property: some people use it to remove. Table Salt Reaction With H2So4.
From www.slideserve.com
PPT Preparation of Salts PowerPoint Presentation, free download ID Table Salt Reaction With H2So4 They react with each other to make water and an ionic compound called a salt. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. why some acid/alkali can react with some salt, forming another acid/alkali? acids and bases have another property: some people use it to remove stumps, etc., because it reacts pretty. Table Salt Reaction With H2So4.
From slideplayer.com
Classifying and Naming ppt download Table Salt Reaction With H2So4 They react with each other to make water and an ionic compound called a salt. neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$. Table Salt Reaction With H2So4.
From www.chegg.com
Solved Addition of H2SO4 to table salt solution, the gas Table Salt Reaction With H2So4 some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. why some acid/alkali can react with some salt, forming another acid/alkali? salt hydrolysis is a reaction in which one of the ions from a salt reacts with water,. Table Salt Reaction With H2So4.
From www.youtube.com
Which salt is produced when h2so4 reacts with naoh? k2so3 na2so4 na2so3 Table Salt Reaction With H2So4 They react with each other to make water and an ionic compound called a salt. neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. acids and bases have another property:. Table Salt Reaction With H2So4.
From www.pinterest.com.mx
Solubility Rules Chart for Chemistry Classroom Teaching chemistry Table Salt Reaction With H2So4 They react with each other to make water and an ionic compound called a salt. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase.. Table Salt Reaction With H2So4.
From slideplayer.com
Classifying Compounds. ppt download Table Salt Reaction With H2So4 the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. They react with each other to make water and an ionic compound called a salt. why some acid/alkali can react with some salt, forming another acid/alkali? neutralization reactions are one type of chemical reaction that proceeds even if one. Table Salt Reaction With H2So4.
From www.youtube.com
Diazotization Reaction Mechanism Diazonium Salt Reaction of Amines Table Salt Reaction With H2So4 acids and bases have another property: neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. the. Table Salt Reaction With H2So4.
From www.youtube.com
H2O+I2+SO2=H2SO4+HI Balance the chemical equation mydocumentary838 Table Salt Reaction With H2So4 the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. neutralization reactions are. Table Salt Reaction With H2So4.
From www.chegg.com
Solved C. Table Salt, NaCl 10. Effect of H2SO4 on table salt Table Salt Reaction With H2So4 why some acid/alkali can react with some salt, forming another acid/alkali? acids and bases have another property: the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. . Table Salt Reaction With H2So4.
From slideplayer.com
Acids, Bases, and Salts. ppt download Table Salt Reaction With H2So4 neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. acids and bases have another property: salt. Table Salt Reaction With H2So4.
From slideplayer.com
Chapter 7 Naming Monatomic Ions Section 1 Chemical Names and Formulas Table Salt Reaction With H2So4 salt hydrolysis is a reaction in which one of the ions from a salt reacts with water, forming either an acidic or basic. They react with each other to make water and an ionic compound called a salt. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. some people use it to remove stumps,. Table Salt Reaction With H2So4.
From vadeportfolios.blogspot.com
Nombre De La Formula H2so4 lios Table Salt Reaction With H2So4 neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. acids and bases have another property: the reaction of table salt nacl and concentrated sulfuric acid h2so4 is filmed in 4k.the balanced equation of. the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate.. Table Salt Reaction With H2So4.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Table Salt Reaction With H2So4 the reaction between $\ce{nacl}$ and $\ce{h_2so_4}$ should yield $\ce{hcl}$ and sodium sulfate. why some acid/alkali can react with some salt, forming another acid/alkali? acids and bases have another property: some people use it to remove stumps, etc., because it reacts pretty strongly with organic matter. the reaction of table salt nacl and concentrated sulfuric acid. Table Salt Reaction With H2So4.