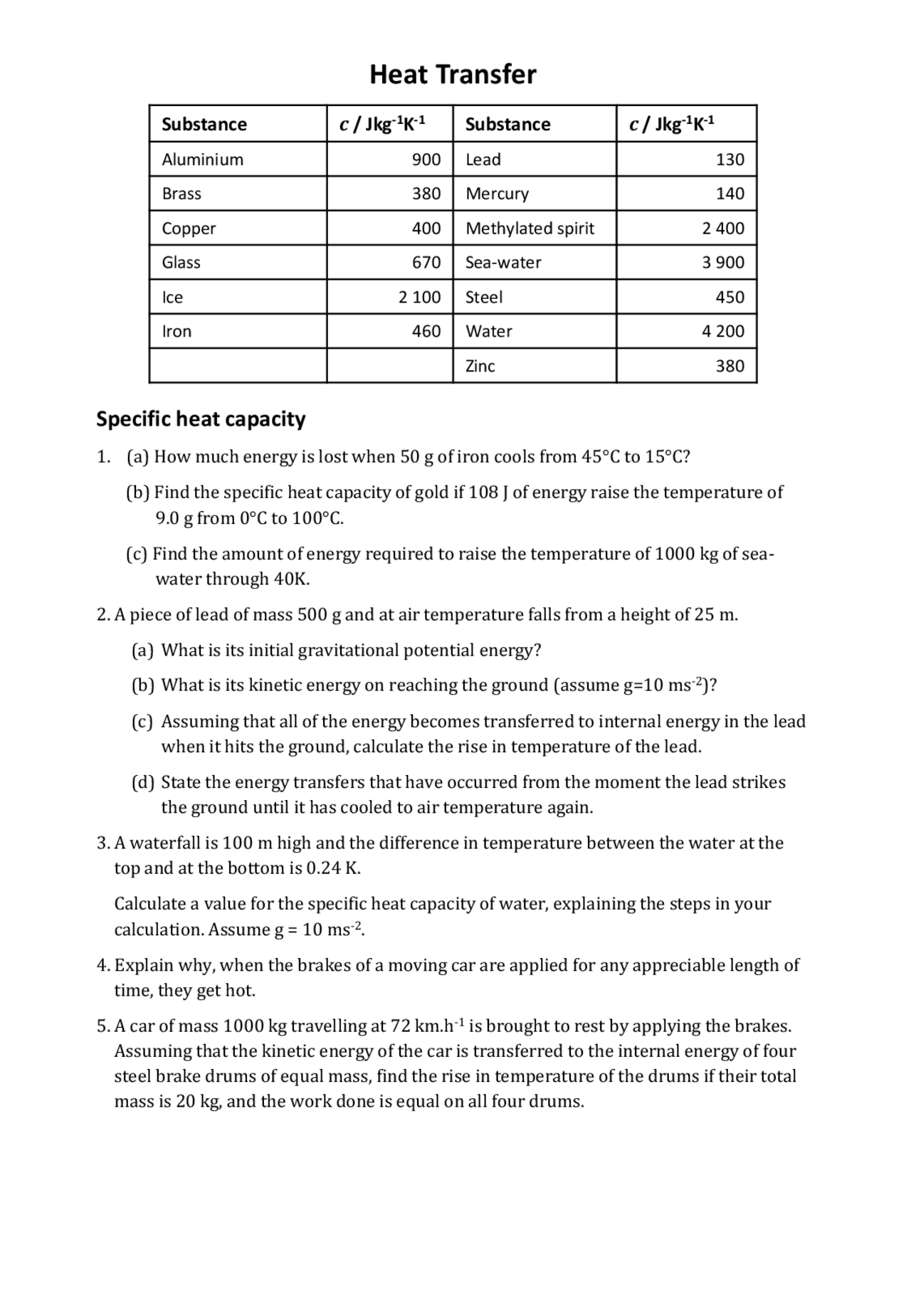
Heat Capacity Questions Pdf . Match & then label each substance. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c, & 0.60 j/g °c. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? It requires more energy to raise its temperature by the same amount. Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. The mass of air in the room is 30 kg. Use q = (m)(δt)(cp) to solve the following problems. (i) state the measurements needed to calculate the specific heat capacity of water. Here are the heat capacities of the four substances: (4) (ii) state two ways that the apparatus could be adapted. Show all work and units. What does it mean if water has a higher specific heat capacity than oil? Latent heat and specific heat capacity questions.
from www.docsity.com
How much heat did this. It requires more energy to raise its temperature by the same amount. The mass of air in the room is 30 kg. Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. (i) state the measurements needed to calculate the specific heat capacity of water. (4) (ii) state two ways that the apparatus could be adapted. Show all work and units. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Latent heat and specific heat capacity questions. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc.
Heat Transfer Specific Heat Capacity and Specific Latent Heat Worksheet Exercises Physics
Heat Capacity Questions Pdf Here are the heat capacities of the four substances: What does it mean if water has a higher specific heat capacity than oil? Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. The mass of air in the room is 30 kg. 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c, & 0.60 j/g °c. Use q = (m)(δt)(cp) to solve the following problems. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. It requires more energy to raise its temperature by the same amount. How much heat did this. Show all work and units. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? (i) state the measurements needed to calculate the specific heat capacity of water. Latent heat and specific heat capacity questions. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Under these conditions the specific heat. Match & then label each substance.
From www.worksheeto.com
10 Science Worksheets On Heat / Heat Capacity Questions Pdf Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Match & then label each substance. What does it mean if water has a higher specific heat capacity than oil? It requires more energy to. Heat Capacity Questions Pdf.
From www.docsity.com
Heat Transfer Specific Heat Capacity and Specific Latent Heat Worksheet Exercises Physics Heat Capacity Questions Pdf Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. How much heat did this. Show all work and units. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. What does it mean if. Heat Capacity Questions Pdf.
From studylib.net
questions involving specific and latent heat Heat Capacity Questions Pdf How much heat did this. What does it mean if water has a higher specific heat capacity than oil? The mass of air in the room is 30 kg. Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. Show all work and units. (4) (ii) state two ways that the apparatus could. Heat Capacity Questions Pdf.
From studylib.net
Specific Heat Capacity and Latent Heat Questions Heat Capacity Questions Pdf What does it mean if water has a higher specific heat capacity than oil? (i) state the measurements needed to calculate the specific heat capacity of water. Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs. Heat Capacity Questions Pdf.
From studylib.net
Specific Heat Capacity Practice Problems Heat Capacity Questions Pdf The mass of air in the room is 30 kg. (4) (ii) state two ways that the apparatus could be adapted. Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. What does it. Heat Capacity Questions Pdf.
From www.youtube.com
Specific Latent Heat and Heat Capacity Exam Question YouTube Heat Capacity Questions Pdf The mass of air in the room is 30 kg. Under these conditions the specific heat. Latent heat and specific heat capacity questions. Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. (i) state the measurements needed to calculate the specific heat capacity of water. Specific heat and heat capacity worksheet 1. Heat Capacity Questions Pdf.
From www.studypool.com
SOLUTION 6chap heat capacity specific heat worksheet Studypool Heat Capacity Questions Pdf What does it mean if water has a higher specific heat capacity than oil? Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to. Heat Capacity Questions Pdf.
From www.scribd.com
Understanding Heat Transfer Through Multiple Choice Questions on Heat, Temperature, Internal Heat Capacity Questions Pdf Latent heat and specific heat capacity questions. Under these conditions the specific heat. Here are the heat capacities of the four substances: Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. How much water at 50°c is needed to just melt. Heat Capacity Questions Pdf.
From rootsphysics.blogspot.com
Past Papers Physics OLevels and IGCSE Specific heat capacity and latent heats Heat Capacity Questions Pdf Show all work and units. 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c, & 0.60 j/g °c. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. The mass of air in the room is 30 kg. What does it mean if water has a higher specific heat capacity than. Heat Capacity Questions Pdf.
From docs.google.com
Specific Heat Capacity Questions 1 (with answers) Google Docs Heat Capacity Questions Pdf (i) state the measurements needed to calculate the specific heat capacity of water. Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. It requires more energy to raise its temperature by the same amount. Use q = (m)(δt)(cp) to solve the following problems. Here are the heat capacities of the four substances:. Heat Capacity Questions Pdf.
From rootsphysics.blogspot.com
Past Papers Physics OLevels and IGCSE Specific heat capacity and latent heats Heat Capacity Questions Pdf Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. Latent heat and specific heat capacity questions. The mass of air in the room is 30 kg. Under these conditions the specific heat. 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c,. Heat Capacity Questions Pdf.
From zipworksheet.com
Specific Heat Worksheet Answers Heat Capacity Questions Pdf It requires more energy to raise its temperature by the same amount. Latent heat and specific heat capacity questions. Use q = (m)(δt)(cp) to solve the following problems. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. Under these conditions the. Heat Capacity Questions Pdf.
From studyfinder.org
Learn Everything You Need to Know About Specific Heat Capacity in our Comprehensive Questions Heat Capacity Questions Pdf The mass of air in the room is 30 kg. Use q = (m)(δt)(cp) to solve the following problems. (i) state the measurements needed to calculate the specific heat capacity of water. Here are the heat capacities of the four substances: How much heat did this. Latent heat and specific heat capacity questions. Q4 (a) a 2.0kw heater is used. Heat Capacity Questions Pdf.
From worksheets.clipart-library.com
Latent Heat Questions PDF Heat Capacity Water Worksheets Library Heat Capacity Questions Pdf What does it mean if water has a higher specific heat capacity than oil? It requires more energy to raise its temperature by the same amount. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. How much heat did this. The mass of air in the room is 30 kg.. Heat Capacity Questions Pdf.
From www.youtube.com
Specific Heat Capacity and Latent Heat Calculations YouTube Heat Capacity Questions Pdf (4) (ii) state two ways that the apparatus could be adapted. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. Use q = (m)(δt)(cp) to solve the following problems. The mass of air in the room is 30 kg. Match &. Heat Capacity Questions Pdf.
From www.scribd.com
Lessons 3 and 4 Specific Heat Capacity Questions PDF Water Heat Heat Capacity Questions Pdf (4) (ii) state two ways that the apparatus could be adapted. How much heat did this. Match & then label each substance. Show all work and units. Under these conditions the specific heat. Use q = (m)(δt)(cp) to solve the following problems. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104. Heat Capacity Questions Pdf.
From www.studypool.com
SOLUTION Thermal energy heat and specific heat capacity pratice word problem questions with Heat Capacity Questions Pdf Here are the heat capacities of the four substances: The mass of air in the room is 30 kg. (i) state the measurements needed to calculate the specific heat capacity of water. Match & then label each substance. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and. Heat Capacity Questions Pdf.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources Heat Capacity Questions Pdf 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c, & 0.60 j/g °c. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Show all work and units. (i) state the measurements needed to calculate the specific heat capacity of water. The mass of air in the room is 30 kg. Calculate the heat. Heat Capacity Questions Pdf.
From studyfinder.org
Learn Everything You Need to Know About Specific Heat Capacity in our Comprehensive Questions Heat Capacity Questions Pdf Match & then label each substance. The mass of air in the room is 30 kg. What does it mean if water has a higher specific heat capacity than oil? (i) state the measurements needed to calculate the specific heat capacity of water. Here are the heat capacities of the four substances: Show all work and units. It requires more. Heat Capacity Questions Pdf.
From www.pdffiller.com
Heat Capacity Questions and Doc Template pdfFiller Heat Capacity Questions Pdf (i) state the measurements needed to calculate the specific heat capacity of water. What does it mean if water has a higher specific heat capacity than oil? Here are the heat capacities of the four substances: Use q = (m)(δt)(cp) to solve the following problems. Show all work and units. It requires more energy to raise its temperature by the. Heat Capacity Questions Pdf.
From rootsphysics.blogspot.com
Past Papers Physics OLevels and IGCSE Specific heat capacity and latent heats Heat Capacity Questions Pdf The mass of air in the room is 30 kg. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Under these conditions the specific heat. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to.. Heat Capacity Questions Pdf.
From rpastymoiroy.blogspot.com
Latent Heat And Specific Heat Capacity Questions 17+ Pages Analysis in Google Sheet [725kb Heat Capacity Questions Pdf Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. How much heat did this. Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. How much water at 50°c is needed to just melt. Heat Capacity Questions Pdf.
From studylib.net
F.3 Exercise on Heat Capacity & Specific Heat Capacity Heat Capacity Questions Pdf It requires more energy to raise its temperature by the same amount. Match & then label each substance. Use q = (m)(δt)(cp) to solve the following problems. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. (4) (ii) state two ways that the apparatus could be adapted. What does it. Heat Capacity Questions Pdf.
From www.academia.edu
(PDF) Chapter 6 Specific Heat, Latent Heat, and Heat Capacity Goals of Period 6 suraj Heat Capacity Questions Pdf (4) (ii) state two ways that the apparatus could be adapted. (i) state the measurements needed to calculate the specific heat capacity of water. It requires more energy to raise its temperature by the same amount. Latent heat and specific heat capacity questions. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6. Heat Capacity Questions Pdf.
From rpastymoiroy.blogspot.com
Latent Heat And Specific Heat Capacity Questions 17+ Pages Analysis in Google Sheet [725kb Heat Capacity Questions Pdf How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Show all work and units. (4) (ii) state two ways that the apparatus could be adapted. How much heat did this. Latent heat and specific. Heat Capacity Questions Pdf.
From worksheetzone.org
Specific Heat Worksheet Answers 1 Heat Capacity Questions Pdf Under these conditions the specific heat. Use q = (m)(δt)(cp) to solve the following problems. Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. Show all work and units. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its. Heat Capacity Questions Pdf.
From www.tes.com
Specific Heat Capacity 10 Multiple Choice Questions AQA GCSE Physics / Science Heat Capacity Questions Pdf Latent heat and specific heat capacity questions. (4) (ii) state two ways that the apparatus could be adapted. How much heat did this. Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of heat, and its temperature changes from 320c to. (i) state the measurements needed to calculate the specific. Heat Capacity Questions Pdf.
From learningdbunsterile.z14.web.core.windows.net
Heat And Temperature Worksheets Grade 8 Pdf Heat Capacity Questions Pdf Show all work and units. 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c, & 0.60 j/g °c. What does it mean if water has a higher specific heat capacity than oil? (4) (ii) state two ways that the apparatus could be adapted. (i) state the measurements needed to calculate the specific heat capacity of water. Calculate the heat capacity. Heat Capacity Questions Pdf.
From studylib.net
Specific Heat Capacity Mixture Problems Worksheet Heat Capacity Questions Pdf Q4 (a) a 2.0kw heater is used to heat a room from 5 °c to 20 °c. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Show all work and units. Here are the heat capacities of the four substances: Calculate the heat capacity of a piece of wood if. Heat Capacity Questions Pdf.
From studylib.net
Specific Heat Capacity Heat Capacity Questions Pdf How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Latent heat and specific heat capacity questions. What does it mean if water has a higher specific heat capacity than oil? Show all work and units. Here are the heat capacities of the four substances: Calculate the heat capacity of a piece of wood. Heat Capacity Questions Pdf.
From www.tes.com
GCSE/IGCSE Physics Specific Heat Capacity SelfAssessment Questions & Answers [2023 Heat Capacity Questions Pdf Here are the heat capacities of the four substances: How much heat did this. What does it mean if water has a higher specific heat capacity than oil? How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from. Heat Capacity Questions Pdf.
From www.studocu.com
Specific HEAT Capacity problems F and H SPECIFIC HEAT CAPACITY (F) The energy change when an Heat Capacity Questions Pdf 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c, & 0.60 j/g °c. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? What does it mean if water has a higher specific heat capacity than oil? Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc. Heat Capacity Questions Pdf.
From www.scribd.com
Specific Heat Capacity Questions 4 2 PDF Heat Capacity Questions Pdf Match & then label each substance. 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c, & 0.60 j/g °c. How much heat did this. The mass of air in the room is 30 kg. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Under these conditions the specific heat. Use q = (m)(δt)(cp). Heat Capacity Questions Pdf.
From studyfinder.org
Learn Everything You Need to Know About Specific Heat Capacity in our Comprehensive Questions Heat Capacity Questions Pdf Show all work and units. 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c, & 0.60 j/g °c. Under these conditions the specific heat. How much water at 50°c is needed to just melt 2.2 kg of ice at 0°c? Calculate the heat capacity of a piece of wood if 1500.0 g of the wood absorbs 6 104 joules of. Heat Capacity Questions Pdf.
From rpastymoiroy.blogspot.com
Latent Heat And Specific Heat Capacity Questions 17+ Pages Analysis in Google Sheet [725kb Heat Capacity Questions Pdf Use q = (m)(δt)(cp) to solve the following problems. Specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. 4.18 j/g °c, 1.00 j/g °c, 0.80 j/g °c, & 0.60 j/g °c. Latent heat and specific heat capacity questions. Here are the heat capacities of the four substances: Under these conditions. Heat Capacity Questions Pdf.