What Does Acid Rain Do To Metals In The Soil . Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. For example, a more acidic. The dissolved aluminum begins to accumulate and. These various chemical changes can contribute to: The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. What does acid rain do to the soil? Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. Soil acidification, the leaching of important chemicals such as calcium and magnesium,.
from www.javatpoint.com
For example, a more acidic. What does acid rain do to the soil? Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. These various chemical changes can contribute to: It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. Soil acidification, the leaching of important chemicals such as calcium and magnesium,. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. Acid rain can dissolve certain more soluble elements from the soil, like aluminum.
Acid Rain Definition JavaTpoint
What Does Acid Rain Do To Metals In The Soil What does acid rain do to the soil? These various chemical changes can contribute to: Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. The dissolved aluminum begins to accumulate and. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. What does acid rain do to the soil? Soil acidification, the leaching of important chemicals such as calcium and magnesium,. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. For example, a more acidic. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to.
From hhsvoyager.org
How does acid rain affect the ecosystem? The Voyager What Does Acid Rain Do To Metals In The Soil The dissolved aluminum begins to accumulate and. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. For example, a more acidic. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. Acid rain can dissolve certain more soluble elements. What Does Acid Rain Do To Metals In The Soil.
From www.teachoo.com
Acid Rain Definition, Causes, Effects Teachoo Concepts What Does Acid Rain Do To Metals In The Soil Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. These various chemical changes can contribute to: It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that. What Does Acid Rain Do To Metals In The Soil.
From rain-acid.weebly.com
Causes Acid Rain What Does Acid Rain Do To Metals In The Soil Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. What does acid rain do to the soil? The dissolved aluminum begins to accumulate and. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. Acid rain penetrates through soil. What Does Acid Rain Do To Metals In The Soil.
From slideplayer.com
Acid Rain. ppt download What Does Acid Rain Do To Metals In The Soil As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. What does acid rain do to the soil? Soil acidification, the leaching of important chemicals such as calcium and magnesium,. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. It. What Does Acid Rain Do To Metals In The Soil.
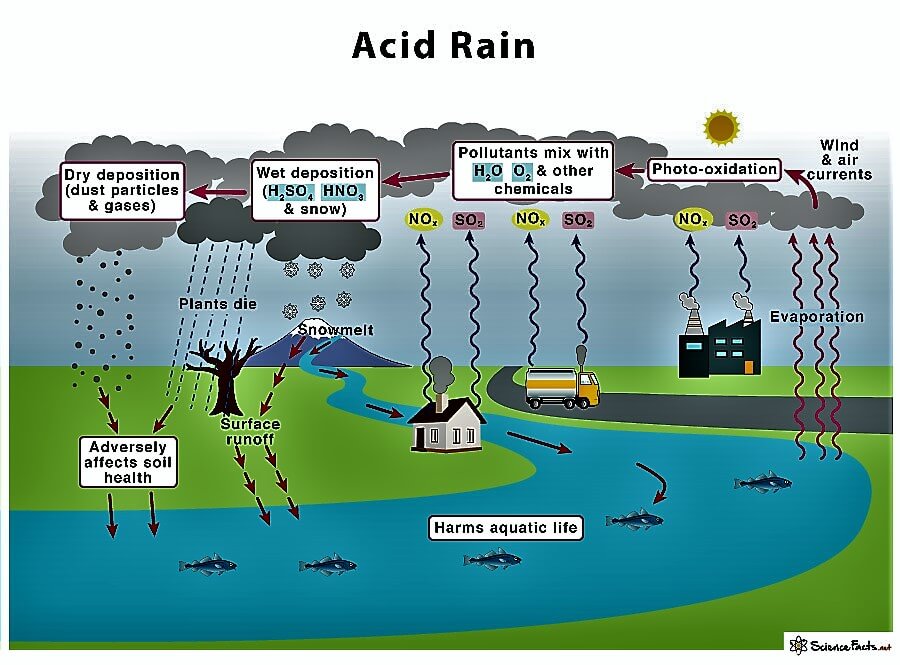
From www.sciencefacts.net
Acid Rain Definition, Causes, Effects, and Solutions What Does Acid Rain Do To Metals In The Soil The dissolved aluminum begins to accumulate and. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. Acid rain can dissolve certain more soluble elements from the soil, like aluminum.. What Does Acid Rain Do To Metals In The Soil.
From www.climateandweather.net
Acid Rain Climate & Weather What Does Acid Rain Do To Metals In The Soil These various chemical changes can contribute to: Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. The dissolved aluminum begins to accumulate and. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. Soil acidification,. What Does Acid Rain Do To Metals In The Soil.
From infogram.com
How acid rain effects soils Infogram What Does Acid Rain Do To Metals In The Soil Soil acidification, the leaching of important chemicals such as calcium and magnesium,. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. The dissolved aluminum begins to. What Does Acid Rain Do To Metals In The Soil.
From www.javatpoint.com
Acid Rain Definition JavaTpoint What Does Acid Rain Do To Metals In The Soil It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. These various chemical changes can contribute to: Soil acidification, the leaching of important chemicals such as calcium and magnesium,. For example, a more acidic. The dissolved aluminum begins to accumulate and. As acid rain moves through the soils, it can strip. What Does Acid Rain Do To Metals In The Soil.
From www.britannica.com
Acid rain Chemistry, Pollutants, Effects Britannica What Does Acid Rain Do To Metals In The Soil Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. For example, a more acidic. Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that. What Does Acid Rain Do To Metals In The Soil.
From www.worldatlas.com
What Is Acid Rain? WorldAtlas What Does Acid Rain Do To Metals In The Soil As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. Acid rain. What Does Acid Rain Do To Metals In The Soil.
From www.vecteezy.com
Diagram showing acid rain pathway on white background 1520150 Vector Art at Vecteezy What Does Acid Rain Do To Metals In The Soil Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. Soil acidification, the leaching of important chemicals such as calcium and magnesium,. For example, a more acidic. Acid rain can. What Does Acid Rain Do To Metals In The Soil.
From www.researchgate.net
Acid rain releases aluminum into the environment (Crisponi et al. 2013) Download Scientific What Does Acid Rain Do To Metals In The Soil Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. Acid rain penetrates through soil particles, washing away. What Does Acid Rain Do To Metals In The Soil.
From plantlet.org
Soil Reaction Importance & Factors Affecting Soil pH Plantlet What Does Acid Rain Do To Metals In The Soil It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. The dissolved aluminum begins to accumulate and. As acid rain moves through the soils, it can strip. What Does Acid Rain Do To Metals In The Soil.
From ian.umces.edu
Causes of acid rain Media Library Integration and Application Network What Does Acid Rain Do To Metals In The Soil Acid rain can dissolve certain more soluble elements from the soil, like aluminum. For example, a more acidic. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. Soil acidification, the leaching of important chemicals such as calcium and magnesium,. Acid rain penetrates through soil particles, washing away the basic cations. What Does Acid Rain Do To Metals In The Soil.
From slideplayer.com
Acid Rain. ppt download What Does Acid Rain Do To Metals In The Soil It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. What does acid rain do to the soil? Soil acidification, the leaching of important chemicals such as calcium. What Does Acid Rain Do To Metals In The Soil.
From www.scienceshorts.com
What Does Acid Rain Do To Rocks What Does Acid Rain Do To Metals In The Soil The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. The dissolved aluminum begins to accumulate and. Soil acidification, the leaching of important chemicals such as calcium and magnesium,. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. It. What Does Acid Rain Do To Metals In The Soil.
From www.thestatesman.com
Acid Rain Causes, consequences and control of the disaster What Does Acid Rain Do To Metals In The Soil What does acid rain do to the soil? The dissolved aluminum begins to accumulate and. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. For example, a more acidic. These various chemical changes can contribute to: Each soil has a buffering capacity, which is the ability of the soil to. What Does Acid Rain Do To Metals In The Soil.
From www.lisbonlx.com
Definition Of Acid Rain Examples and Forms What Does Acid Rain Do To Metals In The Soil Soil acidification, the leaching of important chemicals such as calcium and magnesium,. These various chemical changes can contribute to: What does acid rain do to the soil? Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions,. What Does Acid Rain Do To Metals In The Soil.
From www.adda247.com
Acid Rain Definition, Types, Causes, Effects, Examples What Does Acid Rain Do To Metals In The Soil Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. Soil acidification, the leaching of important chemicals such as calcium and magnesium,. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. As acid rain moves through the soils, it. What Does Acid Rain Do To Metals In The Soil.
From www.slideserve.com
PPT Soil Acidity and pH PowerPoint Presentation, free download ID6597868 What Does Acid Rain Do To Metals In The Soil As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. For example, a more acidic. The dissolved aluminum begins to accumulate and. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. Acid rain can dissolve certain more. What Does Acid Rain Do To Metals In The Soil.
From www.slideshare.net
Acid Rain.... What Does Acid Rain Do To Metals In The Soil The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents. What Does Acid Rain Do To Metals In The Soil.
From infothescienceplanet.blogspot.com
Process of soil formation. What Does Acid Rain Do To Metals In The Soil What does acid rain do to the soil? The dissolved aluminum begins to accumulate and. Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. As acid rain moves through the soils, it. What Does Acid Rain Do To Metals In The Soil.
From www.slideshare.net
Acid Rain.... What Does Acid Rain Do To Metals In The Soil As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. The dissolved aluminum begins to accumulate and. The decrease in ph caused by the acid rain also impacts the concentration of. What Does Acid Rain Do To Metals In The Soil.
From www.breeze-technologies.de
How air pollution causes acid rain Breeze Technologies What Does Acid Rain Do To Metals In The Soil For example, a more acidic. These various chemical changes can contribute to: The dissolved aluminum begins to accumulate and. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. What does acid rain do to the soil? Soil acidification, the leaching of important chemicals such as calcium and magnesium,. The decrease in ph caused. What Does Acid Rain Do To Metals In The Soil.
From pollutantsinthegreatlakes.weebly.com
Acid Rain pollution in the great lakes What Does Acid Rain Do To Metals In The Soil Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Soil acidification, the leaching of important chemicals such as calcium and magnesium,. The dissolved aluminum begins to accumulate and. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. It robs the soil of its essential nutrients such as calcium, releasing. What Does Acid Rain Do To Metals In The Soil.
From muhammadabizard1999.blogspot.com
cause and effect What Does Acid Rain Do To Metals In The Soil As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. For example, a more acidic. The dissolved aluminum begins to accumulate and. The decrease in ph caused by the acid rain. What Does Acid Rain Do To Metals In The Soil.
From acidrain2014.weebly.com
Word Equation What Does Acid Rain Do To Metals In The Soil Acid rain penetrates through soil particles, washing away the basic cations of the soil, which are replaced by other acidic cations. Soil acidification, the leaching of important chemicals such as calcium and magnesium,. What does acid rain do to the soil? It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants.. What Does Acid Rain Do To Metals In The Soil.
From hubbardbrook.org
Ecosystem Effects of Acidic Deposition Hubbard Brook Ecosystem Study What Does Acid Rain Do To Metals In The Soil Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Soil acidification, the leaching of important chemicals such as calcium and magnesium,. What does acid rain do to the soil? The dissolved aluminum begins to accumulate and. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present. What Does Acid Rain Do To Metals In The Soil.
From www.internetgeography.net
What is Acid Rain? Geography What Does Acid Rain Do To Metals In The Soil These various chemical changes can contribute to: For example, a more acidic. The dissolved aluminum begins to accumulate and. The decrease in ph caused by the acid rain also impacts the concentration of different heavy metals that are present in the surrounding water. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions,. What Does Acid Rain Do To Metals In The Soil.
From www.slideserve.com
PPT Acid Rain PowerPoint Presentation, free download ID2409706 What Does Acid Rain Do To Metals In The Soil Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. It robs the soil of its essential nutrients such as calcium, releasing aluminum that prevents water uptake in plants. Soil acidification,. What Does Acid Rain Do To Metals In The Soil.
From www.agric.wa.gov.au
Causes of soil acidity Agriculture and Food What Does Acid Rain Do To Metals In The Soil What does acid rain do to the soil? Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. These various chemical changes can contribute to: The decrease in ph caused by the acid rain also impacts the concentration of different heavy. What Does Acid Rain Do To Metals In The Soil.
From forestrypedia.com
Acid Rain Formation, Effects and Control Measure Forestrypedia What Does Acid Rain Do To Metals In The Soil As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. The dissolved aluminum begins to accumulate and. For example, a more acidic. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. Acid rain penetrates through soil particles, washing away the basic cations of. What Does Acid Rain Do To Metals In The Soil.
From www.elementalchemistry.in
ELEMENTAL CHEMISTRY ACID RAIN What Does Acid Rain Do To Metals In The Soil As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. Each soil has a buffering capacity, which is the ability of the soil to neutralize acids. For example, a more acidic. These various chemical changes can contribute to: What does acid rain do to the soil? The. What Does Acid Rain Do To Metals In The Soil.
From slideplayer.com
Acid Rain. ppt download What Does Acid Rain Do To Metals In The Soil Acid rain can dissolve certain more soluble elements from the soil, like aluminum. For example, a more acidic. As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. The dissolved aluminum begins to accumulate and. Each soil has a buffering capacity, which is the ability of the. What Does Acid Rain Do To Metals In The Soil.
From www.alamy.com
. Effects of acid rain on soil and water . Figure 3. Generalized Relationship Between Soil pH What Does Acid Rain Do To Metals In The Soil As acid rain moves through the soils, it can strip away vital plant nutrients through chemical reactions, thus posing a potential threat to. What does acid rain do to the soil? For example, a more acidic. Acid rain can dissolve certain more soluble elements from the soil, like aluminum. These various chemical changes can contribute to: The decrease in ph. What Does Acid Rain Do To Metals In The Soil.