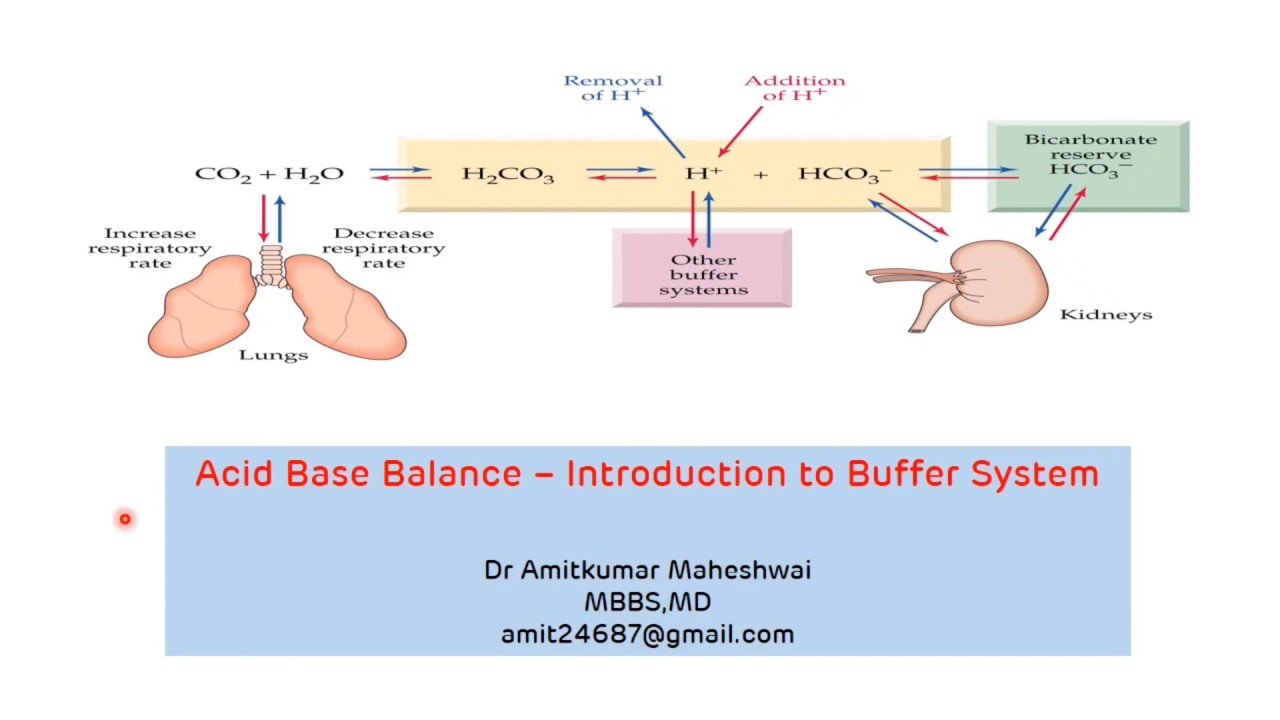
Why Are Buffers Important In Biological Systems . Biological buffers are compounds that help the body maintain a ph around 7.4. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Biochemical experiments routinely require a. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. You should be aware that buffers play a critical role in almost all biochemical systems. Maintenance of biological ph is important. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes.
from studyloadstones.z21.web.core.windows.net
Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. You should be aware that buffers play a critical role in almost all biochemical systems. Biochemical experiments routinely require a. Maintenance of biological ph is important. Biological buffers are compounds that help the body maintain a ph around 7.4. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes.
Why Are Buffers Important To Homeostasis
Why Are Buffers Important In Biological Systems Biological buffers are compounds that help the body maintain a ph around 7.4. Biological buffers are compounds that help the body maintain a ph around 7.4. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. You should be aware that buffers play a critical role in almost all biochemical systems. Biochemical experiments routinely require a. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Maintenance of biological ph is important. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes.
From www.coursehero.com
[Solved] Slide 6 7 Davi 22. Why are buffers important in biological systems?... Course Hero Why Are Buffers Important In Biological Systems You should be aware that buffers play a critical role in almost all biochemical systems. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are compounds that help the body maintain a ph around 7.4. In general, a buffered solution is best able to withstand a change in ph only. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID3105280 Why Are Buffers Important In Biological Systems You should be aware that buffers play a critical role in almost all biochemical systems. Biological buffers are compounds that help the body maintain a ph around 7.4. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. The purpose of a buffer in a biological system. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Why Are Buffers Important In Biological Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Maintenance of biological ph is important. Biological buffers are compounds that help the body maintain a ph around 7.4. Biochemical experiments routinely require a. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph. Why Are Buffers Important In Biological Systems.
From www.slideshare.net
Buffers in biological systems Why Are Buffers Important In Biological Systems You should be aware that buffers play a critical role in almost all biochemical systems. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Biochemical experiments routinely require a. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid. Why Are Buffers Important In Biological Systems.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every biological process is Studocu Why Are Buffers Important In Biological Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. You should be aware that buffers play a critical role in almost all biochemical systems. In general, a buffered. Why Are Buffers Important In Biological Systems.
From www.chegg.com
Solved 13. Buffers are common (and extremely important) in Why Are Buffers Important In Biological Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. You should be aware that buffers play a critical role in almost all biochemical systems. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. The purpose of a buffer. Why Are Buffers Important In Biological Systems.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Why Are Buffers Important In Biological Systems Biological buffers are compounds that help the body maintain a ph around 7.4. Biochemical experiments routinely require a. Maintenance of biological ph is important. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. You should be aware that buffers play a critical role in almost all biochemical systems. The purpose of a. Why Are Buffers Important In Biological Systems.
From agscientific.com
5 Things to Consider When Choosing Biological Buffers Why Are Buffers Important In Biological Systems You should be aware that buffers play a critical role in almost all biochemical systems. Maintenance of biological ph is important. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT 8.3 Bases PowerPoint Presentation, free download ID5917783 Why Are Buffers Important In Biological Systems Biochemical experiments routinely require a. Maintenance of biological ph is important. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. You should be aware that buffers play a critical role in almost all biochemical systems. Biological buffers are compounds that help the body maintain a ph around 7.4. In general, a buffered. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 Why Are Buffers Important In Biological Systems Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Biological buffers are compounds that help the body maintain a ph around 7.4. You should be aware that buffers play a critical role in almost all biochemical systems. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Why Are Buffers Important In Biological Systems.
From www.youtube.com
Role of buffers in biological system YouTube Why Are Buffers Important In Biological Systems Biological buffers are compounds that help the body maintain a ph around 7.4. You should be aware that buffers play a critical role in almost all biochemical systems. Biochemical experiments routinely require a. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffering is important. Why Are Buffers Important In Biological Systems.
From www.youtube.com
CHEM2114 Lecture 13 Buffers in the Biological Systems YouTube Why Are Buffers Important In Biological Systems Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. You should be aware that buffers play a critical role in almost all biochemical systems. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. The buffer. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 Why Are Buffers Important In Biological Systems Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 Why Are Buffers Important In Biological Systems Biological buffers are compounds that help the body maintain a ph around 7.4. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. Biochemical experiments routinely require a. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Why Are Buffers Important In Biological Systems In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. Biological buffers are compounds that help the body maintain a ph around 7.4. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biochemical experiments routinely require a. You should. Why Are Buffers Important In Biological Systems.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies Why Are Buffers Important In Biological Systems In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. Biochemical experiments routinely require a. Biological buffers are compounds that help the body maintain a ph around 7.4. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 Why Are Buffers Important In Biological Systems Biochemical experiments routinely require a. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. You should be aware that buffers play a critical role in almost all biochemical systems. Maintenance of biological ph is important. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation ID2948821 Why Are Buffers Important In Biological Systems The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. You should be aware that buffers play a critical role in almost all biochemical systems. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biochemical experiments routinely require. Why Are Buffers Important In Biological Systems.
From www.chegg.com
Solved 1. What do buffers do and why are they important in Why Are Buffers Important In Biological Systems Biological buffers are compounds that help the body maintain a ph around 7.4. Biochemical experiments routinely require a. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. You should be aware that buffers play a critical role in almost all biochemical systems. Maintenance of biological. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 Why Are Buffers Important In Biological Systems In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. Biological buffers are compounds that help the body maintain a ph around 7.4. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. The purpose of a. Why Are Buffers Important In Biological Systems.
From www.slideshare.net
Buffers in biological systems Why Are Buffers Important In Biological Systems In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. You should be aware that buffers play a critical role in almost all. Why Are Buffers Important In Biological Systems.
From www.chegg.com
Solved 1. What do buffers do and why are they important in Why Are Buffers Important In Biological Systems You should be aware that buffers play a critical role in almost all biochemical systems. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. Biological buffers are compounds that help the body maintain a ph around 7.4. Maintenance of biological ph is important. The purpose of. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Role of Buffers PowerPoint Presentation, free download ID1883450 Why Are Buffers Important In Biological Systems Biological buffers are compounds that help the body maintain a ph around 7.4. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffering is important in living systems. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Why Are Buffers Important In Biological Systems In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Maintenance of biological ph is important. Biological buffers are compounds that help the. Why Are Buffers Important In Biological Systems.
From slideplayer.com
Biology Concepts & Applications ppt download Why Are Buffers Important In Biological Systems Biochemical experiments routinely require a. Biological buffers are compounds that help the body maintain a ph around 7.4. Maintenance of biological ph is important. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. You should be aware that buffers play a critical role in almost all biochemical systems. The. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID3105280 Why Are Buffers Important In Biological Systems In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Biochemical experiments routinely require a. Biological buffers are compounds that help the body maintain a ph around. Why Are Buffers Important In Biological Systems.
From studyloadstones.z21.web.core.windows.net
Why Are Buffers Important To Homeostasis Why Are Buffers Important In Biological Systems Maintenance of biological ph is important. Biological buffers are compounds that help the body maintain a ph around 7.4. Biochemical experiments routinely require a. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Why Are Buffers Important In Biological Systems.
From www.pinterest.com
What are the Buffers and its Importance? This article explains the basic concept of buffers Why Are Buffers Important In Biological Systems You should be aware that buffers play a critical role in almost all biochemical systems. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Maintenance of biological ph. Why Are Buffers Important In Biological Systems.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems. YouTube Why Are Buffers Important In Biological Systems The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Biochemical experiments routinely require a. The buffer systems functioning in blood plasma include plasma proteins, phosphate,. Why Are Buffers Important In Biological Systems.
From www.chegg.com
Solved ID Question 24 Why are buffers important in Why Are Buffers Important In Biological Systems In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. Maintenance of biological ph is important. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. You should be aware that buffers play. Why Are Buffers Important In Biological Systems.
From www.chegg.com
Solved Explain why buffers are important in biological Why Are Buffers Important In Biological Systems Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Biological buffers are compounds that help the body maintain a ph around 7.4. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. Maintenance of biological ph. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation ID315515 Why Are Buffers Important In Biological Systems The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. Biological buffers are compounds that help the body maintain a ph around 7.4. Biochemical experiments routinely require a. Maintenance of biological ph is important. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT UNIT IV PowerPoint Presentation, free download ID4510549 Why Are Buffers Important In Biological Systems Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. In general, a buffered solution is best able to withstand a change in ph only with + 1 ph unit from the pka. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID3105280 Why Are Buffers Important In Biological Systems Maintenance of biological ph is important. The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffering is important in living systems as a means of maintaining a fairly constant internal environment, also known as homeostasis. Biochemical experiments routinely require a. Biological buffers are compounds that help the body maintain a ph around. Why Are Buffers Important In Biological Systems.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 Why Are Buffers Important In Biological Systems The buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Biological buffers are compounds that help the body maintain a ph around 7.4. The purpose of a buffer in a biological system is to maintain intracellular and extracellular ph within a very narrow range and resist changes. In general, a buffered solution is. Why Are Buffers Important In Biological Systems.