Nickel And Oxygen Reaction . Nickel does not react with oxygen, o 2 at room temperature, under normal conditions. When metals react with oxygen, metal oxides are formed. No, n 2 o, n 2 o 3, no 2, n 2 o 5. The speed of the reaction depends on the reactivity of the metal. When heated above 800°c it reacts with oxygen, forming nickel oxide: It interacts with carbon monoxide, forming the poisonous gas carbonyl: Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: 2ni + o₂ → 2nio. All these oxides are gases at room temperature except for n 2 o 5, which is solid. Finely divided nickel can burn, forming nickel(ii) oxide,. Watch this video for a. Reaction of nickel with acids. Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. Ni + 4сo → ni (сo)₄. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form:
from ellaaressims.blogspot.com
No, n 2 o, n 2 o 3, no 2, n 2 o 5. 5ni + 3o₂ → 3nio + ni₂o₃. Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: Reaction of nickel with acids. All these oxides are gases at room temperature except for n 2 o 5, which is solid. Watch this video for a. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: Finely divided nickel can burn, forming nickel(ii) oxide,. Nickel reacts with most acids to produce hydrogen gas and the green ni2+ ion. When metals react with oxygen, metal oxides are formed.
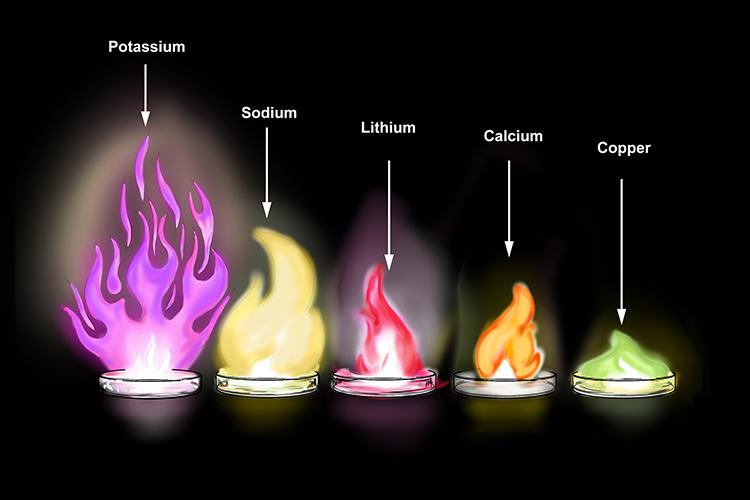
Potassium Reaction With Oxygen EllaaresSims
Nickel And Oxygen Reaction The nitrogen oxides are given below: Ni + 4сo → ni (сo)₄. When heated above 800°c it reacts with oxygen, forming nickel oxide: No, n 2 o, n 2 o 3, no 2, n 2 o 5. Nickel reacts with most acids to produce hydrogen gas and the green ni2+ ion. Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: All of these reactions are endothermic, requiring energy for oxygen to react directly with n 2 (g). It interacts with carbon monoxide, forming the poisonous gas carbonyl: All these oxides are gases at room temperature except for n 2 o 5, which is solid. The speed of the reaction depends on the reactivity of the metal. The nitrogen oxides are given below: Watch this video for a. 5ni + 3o₂ → 3nio + ni₂o₃. Nickel has been found in some metalloproteins that execute catalytic aerobic oxygenation reactions in which.
From www.researchgate.net
(PDF) Control by atomic layer deposition over the chemical composition Nickel And Oxygen Reaction When heated above 800°c it reacts with oxygen, forming nickel oxide: Ni + 4сo → ni (сo)₄. Occurrence nickel is the earth's 22 nd most abundant. No, n 2 o, n 2 o 3, no 2, n 2 o 5. 2ni + o₂ → 2nio. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and. Nickel And Oxygen Reaction.
From www.researchgate.net
Oxygen Reduction Reaction Studies on Pd Coatings Prepared by Galvanic Nickel And Oxygen Reaction The nitrogen oxides are given below: Reaction of nickel with acids. All of these reactions are endothermic, requiring energy for oxygen to react directly with n 2 (g). Occurrence nickel is the earth's 22 nd most abundant. Nickel does not react with oxygen, o 2 at room temperature, under normal conditions. No, n 2 o, n 2 o 3, no. Nickel And Oxygen Reaction.
From www.vedantu.com
Write the formulas for the following compoundsNickel (II) Sulphate Nickel And Oxygen Reaction When metals react with oxygen, metal oxides are formed. 2ni + o₂ → 2nio. Ni + 4сo → ni (сo)₄. The nitrogen oxides are given below: Finely divided nickel can burn, forming nickel(ii) oxide,. Reaction of nickel with acids. 5ni + 3o₂ → 3nio + ni₂o₃. All of these reactions are endothermic, requiring energy for oxygen to react directly with. Nickel And Oxygen Reaction.
From www.bartleby.com
Answered Nickel and carbon monoxide react to… bartleby Nickel And Oxygen Reaction It interacts with carbon monoxide, forming the poisonous gas carbonyl: No, n 2 o, n 2 o 3, no 2, n 2 o 5. 2ni + o₂ → 2nio. Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. Nickel has been found in some metalloproteins that execute catalytic aerobic oxygenation reactions in. Nickel And Oxygen Reaction.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Nickel And Oxygen Reaction Ni + 4сo → ni (сo)₄. When metals react with oxygen, metal oxides are formed. Watch this video for a. Finely divided nickel can burn, forming nickel(ii) oxide,. All these oxides are gases at room temperature except for n 2 o 5, which is solid. Reaction of nickel with acids. Nickel metal dissolves slowly in dilute sulphuric acid to form. Nickel And Oxygen Reaction.
From www.youtube.com
Li + O2=Li2O Balanced EquationLithium +Oxygen=Lithium oxide Balanced Nickel And Oxygen Reaction Finely divided nickel can burn, forming nickel(ii) oxide,. When metals react with oxygen, metal oxides are formed. Ni + 4сo → ni (сo)₄. When heated above 800°c it reacts with oxygen, forming nickel oxide: All of these reactions are endothermic, requiring energy for oxygen to react directly with n 2 (g). Occurrence nickel is the earth's 22 nd most abundant.. Nickel And Oxygen Reaction.
From www.pnas.org
Determining the hydronium pKα at platinum surfaces and the effect on pH Nickel And Oxygen Reaction Nickel reacts with most acids to produce hydrogen gas and the green ni2+ ion. Ni + 4сo → ni (сo)₄. The speed of the reaction depends on the reactivity of the metal. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: All of these reactions are endothermic, requiring energy for oxygen. Nickel And Oxygen Reaction.
From www.researchgate.net
(PDF) Boosting the Electrocatalytic Activity of NickelIron Layered Nickel And Oxygen Reaction The speed of the reaction depends on the reactivity of the metal. All these oxides are gases at room temperature except for n 2 o 5, which is solid. Occurrence nickel is the earth's 22 nd most abundant. 2ni + o₂ → 2nio. When metals react with oxygen, metal oxides are formed. Nickel has been found in some metalloproteins that. Nickel And Oxygen Reaction.
From www.researchgate.net
Schematic representation of energy related applications, i.e., oxygen Nickel And Oxygen Reaction When metals react with oxygen, metal oxides are formed. The nitrogen oxides are given below: When heated above 800°c it reacts with oxygen, forming nickel oxide: Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: Nickel has been found in some metalloproteins that execute catalytic aerobic oxygenation reactions in which. 2ni. Nickel And Oxygen Reaction.
From www.youtube.com
How to Balance Ni2O3 = Ni + O2 YouTube Nickel And Oxygen Reaction Nickel reacts with most acids to produce hydrogen gas and the green ni2+ ion. 2ni + o₂ → 2nio. Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. Nickel has been found in some metalloproteins that execute catalytic aerobic oxygenation reactions in which. When metals react with oxygen, metal oxides are formed.. Nickel And Oxygen Reaction.
From pubs.rsc.org
Nickel foam and stainless steel mesh as electrocatalysts for hydrogen Nickel And Oxygen Reaction All these oxides are gases at room temperature except for n 2 o 5, which is solid. The speed of the reaction depends on the reactivity of the metal. The nitrogen oxides are given below: Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: Reaction of nickel with acids. 2ni + o₂ →. Nickel And Oxygen Reaction.
From www.researchgate.net
(PDF) In situ Electrochemical Formation of CoreShell NickelIron Nickel And Oxygen Reaction Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: All of these reactions are endothermic, requiring energy for oxygen to react directly with n 2 (g). The nitrogen oxides are given below: Ni + 4сo → ni (сo)₄. When metals react with oxygen, metal oxides are formed. Nickel reacts with most acids to. Nickel And Oxygen Reaction.
From www.researchgate.net
(PDF) Oxygen Isotope Labeling Experiments Reveal Different Reaction Nickel And Oxygen Reaction Occurrence nickel is the earth's 22 nd most abundant. Watch this video for a. When metals react with oxygen, metal oxides are formed. Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. The speed of the reaction depends on the reactivity of the metal. 5ni + 3o₂ → 3nio + ni₂o₃. When. Nickel And Oxygen Reaction.
From ecampusontario.pressbooks.pub
4.1 Physical and Chemical Properties Enhanced Introductory College Nickel And Oxygen Reaction The speed of the reaction depends on the reactivity of the metal. 5ni + 3o₂ → 3nio + ni₂o₃. When heated above 800°c it reacts with oxygen, forming nickel oxide: Ni + 4сo → ni (сo)₄. Reaction of nickel with acids. Finely divided nickel can burn, forming nickel(ii) oxide,. Occurrence nickel is the earth's 22 nd most abundant. Nickel reacts. Nickel And Oxygen Reaction.
From www.chegg.com
Solved Nickel and oxygen react to form nickel(II) oxide via Nickel And Oxygen Reaction Reaction of nickel with acids. 5ni + 3o₂ → 3nio + ni₂o₃. Occurrence nickel is the earth's 22 nd most abundant. Nickel has been found in some metalloproteins that execute catalytic aerobic oxygenation reactions in which. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: Nickel metal dissolves slowly in dilute. Nickel And Oxygen Reaction.
From www.researchgate.net
(PDF) Porous Heterogeneous Sulfide Nickel/Nickel Iron Alloy Catalysts Nickel And Oxygen Reaction 2ni + o₂ → 2nio. Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: Occurrence nickel is the earth's 22 nd most abundant. When metals react with oxygen, metal oxides are formed. Finely divided nickel can burn, forming nickel(ii) oxide,. The nitrogen oxides are given below: All of these reactions are endothermic, requiring. Nickel And Oxygen Reaction.
From news.mit.edu
Study unveils details of how a widely used catalyst splits water MIT Nickel And Oxygen Reaction Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: No, n 2 o, n 2 o 3, no 2, n 2 o 5. When metals react with oxygen, metal oxides are formed. All these oxides are gases at room temperature except for n 2 o 5, which is solid. When heated above 800°c. Nickel And Oxygen Reaction.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Nickel And Oxygen Reaction Nickel has been found in some metalloproteins that execute catalytic aerobic oxygenation reactions in which. No, n 2 o, n 2 o 3, no 2, n 2 o 5. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: Nickel does not react with oxygen, o 2 at room temperature, under normal. Nickel And Oxygen Reaction.
From www.victoriana.com
Belästigung Welt Albtraum raney nickel reduction mechanism Mürrisch Nickel And Oxygen Reaction When metals react with oxygen, metal oxides are formed. Nickel has been found in some metalloproteins that execute catalytic aerobic oxygenation reactions in which. 2ni + o₂ → 2nio. All of these reactions are endothermic, requiring energy for oxygen to react directly with n 2 (g). No, n 2 o, n 2 o 3, no 2, n 2 o 5.. Nickel And Oxygen Reaction.
From www.mdpi.com
Oxygen Free FullText Bifunctional Catalytic Activity of γNiOOH Nickel And Oxygen Reaction Watch this video for a. The speed of the reaction depends on the reactivity of the metal. All these oxides are gases at room temperature except for n 2 o 5, which is solid. When heated above 800°c it reacts with oxygen, forming nickel oxide: Reaction of nickel with acids. 2ni + o₂ → 2nio. Bunsenite — the mineralogical form. Nickel And Oxygen Reaction.
From www.numerade.com
SOLVED Phosphurus( P4) and oxygen react to produce diphosphorus Nickel And Oxygen Reaction When heated above 800°c it reacts with oxygen, forming nickel oxide: 5ni + 3o₂ → 3nio + ni₂o₃. Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. Nickel has been found in some metalloproteins that execute catalytic aerobic oxygenation reactions in which. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder. Nickel And Oxygen Reaction.
From www.bajeczneobrazy.pl
Chemical reaction model of creating new compounds. Hydrogen and Oxygen Nickel And Oxygen Reaction Reaction of nickel with acids. The nitrogen oxides are given below: When metals react with oxygen, metal oxides are formed. Ni + 4сo → ni (сo)₄. Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: Finely divided nickel can burn, forming nickel(ii) oxide,. When heated above 800°c it reacts with oxygen, forming nickel. Nickel And Oxygen Reaction.
From www.researchgate.net
(PDF) HeteroatomDoped Nickel Sulfide for Efficient Electrochemical Nickel And Oxygen Reaction Occurrence nickel is the earth's 22 nd most abundant. The speed of the reaction depends on the reactivity of the metal. When heated above 800°c it reacts with oxygen, forming nickel oxide: Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: Ni + 4сo → ni (сo)₄. It interacts with carbon. Nickel And Oxygen Reaction.
From ellaaressims.blogspot.com
Potassium Reaction With Oxygen EllaaresSims Nickel And Oxygen Reaction The nitrogen oxides are given below: When metals react with oxygen, metal oxides are formed. No, n 2 o, n 2 o 3, no 2, n 2 o 5. All these oxides are gases at room temperature except for n 2 o 5, which is solid. Nickel does not react with oxygen, o 2 at room temperature, under normal conditions.. Nickel And Oxygen Reaction.
From www.researchgate.net
(PDF) Electrodeposition of nickeliron on stainless steel as an Nickel And Oxygen Reaction Finely divided nickel can burn, forming nickel(ii) oxide,. No, n 2 o, n 2 o 3, no 2, n 2 o 5. Occurrence nickel is the earth's 22 nd most abundant. Reaction of nickel with acids. Nickel does not react with oxygen, o 2 at room temperature, under normal conditions. Nickel reacts with most acids to produce hydrogen gas and. Nickel And Oxygen Reaction.
From www.numerade.com
SOLVED Solid nickel reacts with aqueous lead (II) nitrate to form Nickel And Oxygen Reaction 2ni + o₂ → 2nio. Reaction of nickel with acids. The nitrogen oxides are given below: Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. When heated above 800°c it reacts with oxygen, forming nickel oxide: The speed of the reaction depends on the reactivity of the metal. Watch this video for. Nickel And Oxygen Reaction.
From www2.mdpi.com
Oxygen Free FullText Bifunctional Catalytic Activity of γNiOOH Nickel And Oxygen Reaction Watch this video for a. No, n 2 o, n 2 o 3, no 2, n 2 o 5. Nickel reacts with most acids to produce hydrogen gas and the green ni2+ ion. Occurrence nickel is the earth's 22 nd most abundant. 5ni + 3o₂ → 3nio + ni₂o₃. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites. Nickel And Oxygen Reaction.
From www.researchgate.net
Solubility of oxygen in solid nickel at high temperature ͑ literature Nickel And Oxygen Reaction Watch this video for a. Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: Nickel does not react with oxygen, o 2 at room temperature, under normal conditions. The nitrogen oxides are given below:. Nickel And Oxygen Reaction.
From www.researchgate.net
(PDF) PostSynthetic Treatment of NickelIron Layered Double Hydroxides Nickel And Oxygen Reaction The speed of the reaction depends on the reactivity of the metal. Occurrence nickel is the earth's 22 nd most abundant. When metals react with oxygen, metal oxides are formed. Finely divided nickel can burn, forming nickel(ii) oxide,. Reaction of nickel with acids. No, n 2 o, n 2 o 3, no 2, n 2 o 5. Nickel does not. Nickel And Oxygen Reaction.
From www.thesciencehive.co.uk
Group 2* — the science sauce Nickel And Oxygen Reaction All these oxides are gases at room temperature except for n 2 o 5, which is solid. Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. The speed of the reaction depends on the reactivity of the metal. 2ni + o₂ → 2nio. All of these reactions are endothermic, requiring energy for. Nickel And Oxygen Reaction.
From www.researchgate.net
Oxygen evolution reaction (OER) polarization curves of (a) FC, (b Nickel And Oxygen Reaction 2ni + o₂ → 2nio. Nickel does not react with oxygen, o 2 at room temperature, under normal conditions. The nitrogen oxides are given below: Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: All of these reactions are endothermic, requiring energy for oxygen to react directly with n 2 (g). The speed. Nickel And Oxygen Reaction.
From www.pnas.org
Constructing nickeliron oxyhydroxides integrated with iron oxides by Nickel And Oxygen Reaction Nickel reacts with most acids to produce hydrogen gas and the green ni2+ ion. Nickel does not react with oxygen, o 2 at room temperature, under normal conditions. Occurrence nickel is the earth's 22 nd most abundant. Nickel has been found in some metalloproteins that execute catalytic aerobic oxygenation reactions in which. Finely divided nickel can burn, forming nickel(ii) oxide,.. Nickel And Oxygen Reaction.
From www.chegg.com
Solved At a certain temperature this reaction follows Nickel And Oxygen Reaction Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: 2ni + o₂ → 2nio. No, n 2 o, n 2 o 3, no 2, n 2 o 5. All of these reactions are endothermic, requiring. Nickel And Oxygen Reaction.
From pubs.acs.org
An Unconventional Iron Nickel Catalyst for the Oxygen Evolution Nickel And Oxygen Reaction Nickel metal dissolves slowly in dilute sulphuric acid to form solutions containing the aquated ni (ii) ion. When heated above 800°c it reacts with oxygen, forming nickel oxide: Bunsenite — the mineralogical form of nio [wikimedia] nickel powder ignites spontaneously in air and nickel oxides form: Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1. Nickel And Oxygen Reaction.
From www.researchgate.net
Reactions performed during the nickel electroplating process Download Nickel And Oxygen Reaction Nickel reacts with most acids to produce hydrogen gas and the green ni2+ ion. Finely divided nickel can burn, forming nickel(ii) oxide,. The speed of the reaction depends on the reactivity of the metal. Nickel does not react with oxygen, o 2 at room temperature, under normal conditions. It interacts with carbon monoxide, forming the poisonous gas carbonyl: 5ni +. Nickel And Oxygen Reaction.