Medical Device Regulations Uk 2002 . These regulations contain the legislative measures necessary for the implementation of three. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. mhra is the designated authority that administers and enforces the law on medical devices in the uk. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the.
from www.bsigroup.com
uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. mhra is the designated authority that administers and enforces the law on medical devices in the uk. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. These regulations contain the legislative measures necessary for the implementation of three.
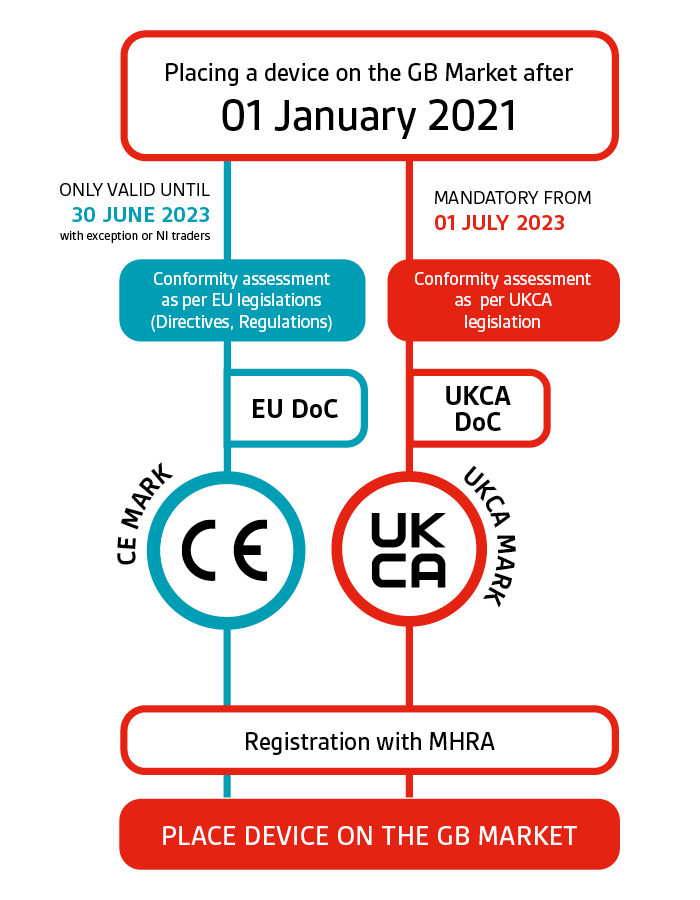
UKCA marking for medical devices certification BSI
Medical Device Regulations Uk 2002 uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. mhra is the designated authority that administers and enforces the law on medical devices in the uk. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. These regulations contain the legislative measures necessary for the implementation of three. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024.
From www.ignitec.com
UK medical device regulations glossary What every medical... Medical Device Regulations Uk 2002 These regulations contain the legislative measures necessary for the implementation of three. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024.. Medical Device Regulations Uk 2002.
From www.tuvsud.cn
Infographic The In Vitro Diagnostic Medical Device Regulation TÜV南德 Medical Device Regulations Uk 2002 uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. These regulations contain the legislative measures necessary for the implementation of three. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. the medical devices. Medical Device Regulations Uk 2002.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Regulations Uk 2002 Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. mhra is the. Medical Device Regulations Uk 2002.
From www.bsigroup.com
UKCA marking for medical devices certification BSI Medical Device Regulations Uk 2002 mhra is the designated authority that administers and enforces the law on medical devices in the uk. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. These regulations contain the legislative measures. Medical Device Regulations Uk 2002.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Medical Device Regulations Uk 2002 Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the.. Medical Device Regulations Uk 2002.
From www.castoredc.com
Overview of EU Medical Device Regulations (MDR) Medical Device Regulations Uk 2002 These regulations contain the legislative measures necessary for the implementation of three. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. the medical devices regulations 2002 is up. Medical Device Regulations Uk 2002.
From omcmedical.com
4 Things about Medical Device Regulation in Europe OMC Medical Limited Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. mhra is the designated authority that administers and enforces the law on medical devices in the uk. These regulations. Medical Device Regulations Uk 2002.
From eurointervention.pcronline.com
Medical device regulation in Europe what is changing and how can I Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. mhra is the designated authority that administers and enforces the law on medical devices in. Medical Device Regulations Uk 2002.
From www.med-technews.com
The impact of new European Medical Device Regulations MedTech Innovation Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. These regulations contain the legislative measures necessary for the implementation of three. mhra is the designated authority that administers and enforces the law on medical devices in the uk. uk law specifies requirements that. Medical Device Regulations Uk 2002.
From omcmedical.com
Classification of Medical Devices Based on UK MDR 2002 Medical Device Regulations Uk 2002 uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. mhra is the designated authority that administers and enforces the law on medical devices in the uk. These regulations contain the legislative measures. Medical Device Regulations Uk 2002.
From www.studocu.com
The Medical Devices Regulation in the UK The Medical Devices Medical Device Regulations Uk 2002 Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. These regulations contain the legislative measures necessary for the implementation of three. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. the medical devices regulations 2002 is up. Medical Device Regulations Uk 2002.
From www.complianceandrisks.com
Medical Device Regulations UK Compliance & Risks Medical Device Regulations Uk 2002 Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. These regulations contain the legislative measures necessary for the implementation of three. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. mhra is the designated authority that administers and enforces the law. Medical Device Regulations Uk 2002.
From info.dicksondata.com
INFOGRAPHIC History of Medical Device Regulation Medical Device Regulations Uk 2002 These regulations contain the legislative measures necessary for the implementation of three. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024.. Medical Device Regulations Uk 2002.
From www.tuvsud.com
Infographic The Medical Device Regulation TÜV SÜD Medical Device Regulations Uk 2002 the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. the medical devices regulations 2002 is up to date with all changes known to be. Medical Device Regulations Uk 2002.
From www.youtube.com
Medical Device Usability Highlights of European Regulations and the Medical Device Regulations Uk 2002 These regulations contain the legislative measures necessary for the implementation of three. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. the medical devices regulations 2002 is up. Medical Device Regulations Uk 2002.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Regulations Uk 2002 These regulations contain the legislative measures necessary for the implementation of three. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. the medical devices. Medical Device Regulations Uk 2002.
From blog.cosmotrace.com
Medical Devices Regulations (MDR) Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as.. Medical Device Regulations Uk 2002.
From www.presentationeze.com
Medical Device Regulation.PresentationEZE Medical Device Regulations Uk 2002 These regulations contain the legislative measures necessary for the implementation of three. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. uk law specifies requirements that legal manufacturers of medical devices must. Medical Device Regulations Uk 2002.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Medical Device Regulations Uk 2002 the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024.. Medical Device Regulations Uk 2002.
From blog.cosmotrace.com
EU Medical Devices Regulations Medical Device Regulations Uk 2002 uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. These regulations contain the legislative measures necessary for the implementation of three. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. the medical devices. Medical Device Regulations Uk 2002.
From www.pathologyinpractice.com
Implementation of future Medical Device Regulations delayed by 12 months Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. the medical devices regulations 2002 is up to date with all changes known to be. Medical Device Regulations Uk 2002.
From www.gov.uk
Factsheet medical devices overview GOV.UK Medical Device Regulations Uk 2002 uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. These regulations contain the legislative measures necessary for the implementation of three. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. mhra is the. Medical Device Regulations Uk 2002.
From www.researchsolutions.com
European Medical Device Regulation Guide to simplify compliance 2021 Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the government has put in place legislation that amends the medical device regulations 2002 (si. Medical Device Regulations Uk 2002.
From www.ignitec.com
How does UK medical devices regulations differ from EU MDR Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. These regulations contain the legislative measures necessary for the implementation of three. mhra is the. Medical Device Regulations Uk 2002.
From apacmed.glueup.com
Understanding Europe's Medical Device Regulation APACMed on Glue Up Medical Device Regulations Uk 2002 These regulations contain the legislative measures necessary for the implementation of three. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the medical devices regulations 2002 is up to date with all. Medical Device Regulations Uk 2002.
From medphanut.com
Certificates of Compliance or Attestation of Conformity have no legal Medical Device Regulations Uk 2002 mhra is the designated authority that administers and enforces the law on medical devices in the uk. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. These regulations contain the legislative measures necessary for the implementation of three. Complying with uk law (uk mdr 2002) uk law specifies. Medical Device Regulations Uk 2002.
From www.goodreads.com
Fundamentals of Medical Device Regulations by Various Medical Device Regulations Uk 2002 These regulations contain the legislative measures necessary for the implementation of three. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. uk law specifies requirements that legal manufacturers. Medical Device Regulations Uk 2002.
From www.raps.org
Here’s what’s new in Fundamentals of Medical Device Regulations, Fifth Medical Device Regulations Uk 2002 uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. These regulations contain the legislative measures necessary for the implementation of three. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. the government has. Medical Device Regulations Uk 2002.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Regulations Uk 2002 uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. mhra is the designated authority that administers and enforces the law on medical devices in the uk. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. the medical devices regulations 2002. Medical Device Regulations Uk 2002.
From www.scribd.com
Therapeutic Goods (Medical Devices) Regulations 2002 PDF Medical Medical Device Regulations Uk 2002 These regulations contain the legislative measures necessary for the implementation of three. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. mhra is the. Medical Device Regulations Uk 2002.
From security.cybellum.com
Intro to Medical Device Standards & Regulations Cybellum Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 19 may 2024. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. the medical devices regulations 2002 is up to date with all changes known to be. Medical Device Regulations Uk 2002.
From www.amazon.co.uk
The Survival Guide to EU Medical Device Regulations Amazon.co.uk Medical Device Regulations Uk 2002 mhra is the designated authority that administers and enforces the law on medical devices in the uk. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as. the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26. Medical Device Regulations Uk 2002.
From www.gov.uk
Medical Device “Certificates of Compliance” / “Attestation of Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. the government has put in place legislation that amends the medical device regulations 2002 (si 2002 no 618, as.. Medical Device Regulations Uk 2002.
From crfweb.com
Medical Device Regulations Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the government has put in place legislation that amends the medical device regulations 2002 (si. Medical Device Regulations Uk 2002.
From www.fira.co.uk
FIRA Medical Devices Regulations (UK MDR 2002) Requirements for… Medical Device Regulations Uk 2002 the medical devices regulations 2002 is up to date with all changes known to be in force on or before 26 september 2024. Complying with uk law (uk mdr 2002) uk law specifies requirements that legal manufacturers of medical. mhra is the designated authority that administers and enforces the law on medical devices in the uk. the. Medical Device Regulations Uk 2002.