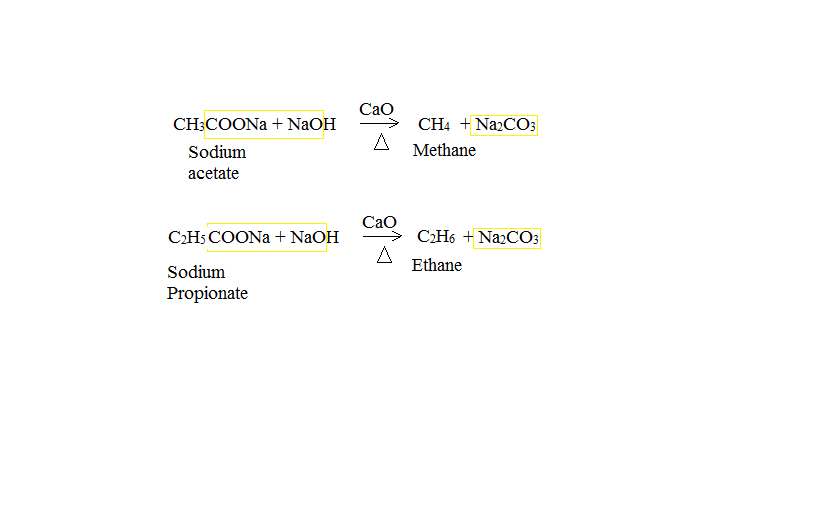
Preparation Of Methane Gas In Laboratory . This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. • liquefied gases are partially liquid at normal temperature and charge pressure. There are three major groups of compressed gases stored in cylinders. This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. Decarboxylation of sodium acetate :
from icsechemistry16.blogspot.com
Decarboxylation of sodium acetate : This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. • liquefied gases are partially liquid at normal temperature and charge pressure. There are three major groups of compressed gases stored in cylinders.
decarboxylation
Preparation Of Methane Gas In Laboratory Decarboxylation of sodium acetate : The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. • liquefied gases are partially liquid at normal temperature and charge pressure. There are three major groups of compressed gases stored in cylinders. This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. Decarboxylation of sodium acetate :
From askfilo.com
12.14.3 Laboratory preparation of methane Reactants Sodium ethanoate (s.. Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. Decarboxylation of sodium acetate : 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. This. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
Methane production Biogas plant Working Mechanism Methanogens Preparation Of Methane Gas In Laboratory This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. This process involves heating sodium acetate with soda lime (a mixture of naoh and. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
Give chemical equations for The laboratory preparation of methane from Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. This process involves heating sodium acetate with soda lime (a mixture of naoh and. Preparation Of Methane Gas In Laboratory.
From flaringmethanetoolkit.com
Composition Gas Chromatograph Methane Flaring Toolkit Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. Decarboxylation of sodium acetate : • liquefied gases are partially liquid at normal temperature and charge pressure. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with. Preparation Of Methane Gas In Laboratory.
From www.pw.live
Hydrocarbon Carbon and its compound class 8 PW Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. Decarboxylation of sodium acetate : This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). • liquefied gases are partially liquid at normal temperature and charge pressure. This laboratory experiment. Preparation Of Methane Gas In Laboratory.
From www.alamy.com
Scientific Designing of Preparation of Methane Gas. Colorful Symbols Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. • liquefied gases are partially liquid at normal temperature and charge pressure. 2.9.8 describe. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
preparation of Methan (Marsh gas) lab procces diagram YouTube Preparation Of Methane Gas In Laboratory This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
laboratory preparation of pure methane, YouTube Preparation Of Methane Gas In Laboratory This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. There are three major groups of compressed gases stored in cylinders. Decarboxylation of sodium acetate : • liquefied gases are partially liquid at normal temperature and charge pressure. The preparation of methane can be achieved through various methods, among. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
Preparation of Methane Gas I XII Chemistry YouTube Preparation Of Methane Gas In Laboratory Decarboxylation of sodium acetate : This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). There are three major groups of compressed gases stored in cylinders. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. The preparation of methane can be achieved through. Preparation Of Methane Gas In Laboratory.
From icsechemistry16.blogspot.com
decarboxylation Preparation Of Methane Gas In Laboratory There are three major groups of compressed gases stored in cylinders. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
Preparation of Methane, Chemistry Lecture Sabaq.pk YouTube Preparation Of Methane Gas In Laboratory Decarboxylation of sodium acetate : There are three major groups of compressed gases stored in cylinders. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. • liquefied gases are partially liquid at normal temperature and charge pressure. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas. Preparation Of Methane Gas In Laboratory.
From www.studypool.com
SOLUTION Preparation of methane in the laboratory class 8 cbse science Preparation Of Methane Gas In Laboratory This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. Decarboxylation of sodium acetate : 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. This process involves heating sodium. Preparation Of Methane Gas In Laboratory.
From www.alamy.com
Scientific Designing of Preparation of Methane Gas. Colorful Symbols Preparation Of Methane Gas In Laboratory There are three major groups of compressed gases stored in cylinders. This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. Decarboxylation of sodium acetate :. Preparation Of Methane Gas In Laboratory.
From www.solutionspile.com
[Solved] 2. Methane gas can be produced in a laboratory wi Preparation Of Methane Gas In Laboratory This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). There are three major groups of compressed gases stored in cylinders. Decarboxylation of sodium acetate : The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. This laboratory experiment demonstrates. Preparation Of Methane Gas In Laboratory.
From methanegaskimawashi.blogspot.com
Methane Gas Laboratory Preparation Of Methane Gas Preparation Of Methane Gas In Laboratory • liquefied gases are partially liquid at normal temperature and charge pressure. This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). There are three major groups of compressed gases stored in cylinders. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of. Preparation Of Methane Gas In Laboratory.
From www.numerade.com
SOLVED 1. Illustrate the production of biogas. 2. Describe the Preparation Of Methane Gas In Laboratory 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. • liquefied gases are partially liquid at normal temperature and charge pressure. The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the. Preparation Of Methane Gas In Laboratory.
From www.dreamstime.com
Preparation of Oxygen Gas in Laboratory with the Help of Potassium Preparation Of Methane Gas In Laboratory This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. There are three major groups of compressed gases stored in cylinders. • liquefied gases are partially liquid at normal temperature. Preparation Of Methane Gas In Laboratory.
From www.scribd.com
Preparation of Methane Gas PDF Preparation Of Methane Gas In Laboratory This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. • liquefied gases are partially liquid at normal temperature and charge pressure. Decarboxylation of. Preparation Of Methane Gas In Laboratory.
From chemistnotes.com
Laboratory Preparation of Methane Alkane Chemistry Notes Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. • liquefied gases are partially liquid at normal temperature and charge pressure. Decarboxylation of. Preparation Of Methane Gas In Laboratory.
From www.mdpi.com
Efficient Storage of Methane in Hydrate Form Using Soybean Powder Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. This process involves heating sodium acetate with soda lime (a mixture of naoh and. Preparation Of Methane Gas In Laboratory.
From www.researchgate.net
Schematic diagram of steam methane reforming process. Download Preparation Of Methane Gas In Laboratory • liquefied gases are partially liquid at normal temperature and charge pressure. Decarboxylation of sodium acetate : This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. 2.9.8 describe the laboratory preparation and collection. Preparation Of Methane Gas In Laboratory.
From www.studypool.com
SOLUTION Chemistry of methane(introduction, structure of methane Preparation Of Methane Gas In Laboratory Decarboxylation of sodium acetate : There are three major groups of compressed gases stored in cylinders. This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). • liquefied gases are partially liquid at normal temperature and charge pressure. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid,. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
Preparation Of Methane YouTube Preparation Of Methane Gas In Laboratory • liquefied gases are partially liquid at normal temperature and charge pressure. There are three major groups of compressed gases stored in cylinders. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. This laboratory experiment demonstrates a general approach to. Preparation Of Methane Gas In Laboratory.
From www.doubtnut.com
How is methane gas prepared in laboratory Preparation Of Methane Gas In Laboratory Decarboxylation of sodium acetate : This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. There are three major groups of compressed gases stored in cylinders. The preparation of methane can be achieved through. Preparation Of Methane Gas In Laboratory.
From chem.libretexts.org
8.5 Gas Laws Chemistry LibreTexts Preparation Of Methane Gas In Laboratory Decarboxylation of sodium acetate : This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. There are three major groups. Preparation Of Methane Gas In Laboratory.
From labonlaptop.com
labOnLaptop Store for India Virtual Lab Educational Software Preparation Of Methane Gas In Laboratory There are three major groups of compressed gases stored in cylinders. This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). Decarboxylation of sodium acetate : This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. 2.9.8 describe the laboratory preparation and collection of. Preparation Of Methane Gas In Laboratory.
From www.pinterest.com
Preparation of methane gasPreparationmethanegas Methane Preparation Of Methane Gas In Laboratory • liquefied gases are partially liquid at normal temperature and charge pressure. Decarboxylation of sodium acetate : The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
preparation of methane in laboratory from sodium acetate and soda lime Preparation Of Methane Gas In Laboratory This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are. Preparation Of Methane Gas In Laboratory.
From www.researchgate.net
Schematic of experimental apparatus for enrichment of methane Preparation Of Methane Gas In Laboratory 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. The preparation of methane can be achieved through various methods,. Preparation Of Methane Gas In Laboratory.
From www.studypool.com
SOLUTION Chemistry of methane(introduction, structure of methane Preparation Of Methane Gas In Laboratory 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. There are three major groups of compressed gases stored in cylinders. • liquefied gases are partially liquid at normal temperature and charge pressure. Decarboxylation of sodium acetate : The preparation of. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
Write a balanced chemical equation for the preparation of Methane from Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. Decarboxylation of sodium acetate : •. Preparation Of Methane Gas In Laboratory.
From chemistnotes.com
Laboratory Preparation of Methane Alkane Chemistry Notes Preparation Of Methane Gas In Laboratory This process involves heating sodium acetate with soda lime (a mixture of naoh and cao). There are three major groups of compressed gases stored in cylinders. This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. Decarboxylation of sodium acetate : 2.9.8 describe the laboratory preparation and collection of. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
LABORATORY PREPARATION OF METHANE AND ETHANE YouTube Preparation Of Methane Gas In Laboratory This laboratory experiment demonstrates a general approach to the methanation of syngas in a fixed bed reactor with online chromatographic monitoring. There are three major groups of compressed gases stored in cylinders. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and. Preparation Of Methane Gas In Laboratory.
From www.researchgate.net
A) Methane synthesis through renewablespowered electrochemical Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. There are three major groups of. Preparation Of Methane Gas In Laboratory.
From www.youtube.com
Laboratory preparation of methane how to make methane gas in Preparation Of Methane Gas In Laboratory The preparation of methane can be achieved through various methods, among which the decarboxylation of sodium acetate and the hydrolysis of aluminium carbide are notable. 2.9.8 describe the laboratory preparation and collection of carbon dioxide gas using calcium carbonate and hydrochloric acid, and recall the uses of carbon dioxide in fizzy drinks and fire. There are three major groups of. Preparation Of Methane Gas In Laboratory.