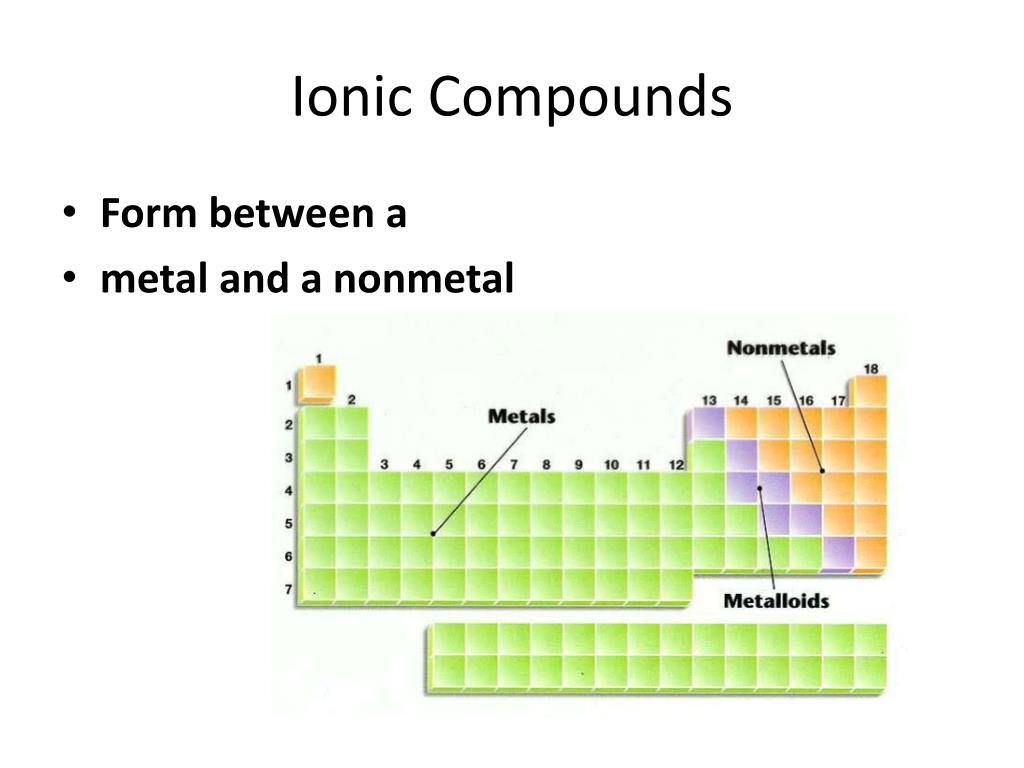
Are Ionic Bonds Metal And Nonmetal . a compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us. Ionic bonds are formed between cations and anions. A metal (which forms the cations) and a nonmetal (which forms the. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. binary ionic compounds are composed of just two elements: ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals.
from www.slideserve.com
ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. The periodic table can help us. a compound that contains ions and is held together by ionic bonds is called an ionic compound. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non.
PPT Ion Charge and the Formulas of Ionic Compounds PowerPoint Presentation ID2068191
Are Ionic Bonds Metal And Nonmetal A metal (which forms the cations) and a nonmetal (which forms the. The periodic table can help us. a compound that contains ions and is held together by ionic bonds is called an ionic compound. Ionic bonds are formed between cations and anions. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the.
From www.youtube.com
Predicting bond type (metals vs. nonmetals) AP Chemistry Khan Academy YouTube Are Ionic Bonds Metal And Nonmetal A metal (which forms the cations) and a nonmetal (which forms the. binary ionic compounds are composed of just two elements: Ionic bonds are formed between cations and anions. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. ionic bonding results in compounds known as ionic, or electrovalent,. Are Ionic Bonds Metal And Nonmetal.
From scientifictutor.org
Chem Covalent, Ionic, and Metallic Bonds (Intramolecular Forces) Scientific Tutor Are Ionic Bonds Metal And Nonmetal binary ionic compounds are composed of just two elements: Ionic bonds are formed between cations and anions. A metal (which forms the cations) and a nonmetal (which forms the. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. The periodic table can help us. a compound that. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Chemistry Unit 4 Bonding ppt download Are Ionic Bonds Metal And Nonmetal a compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT CHAPTER 2 A tomic structure and interatomic bonding PowerPoint Presentation ID5479764 Are Ionic Bonds Metal And Nonmetal A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. A metal (which forms the cations) and a nonmetal (which forms the. binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Ion Charge and the Formulas of Ionic Compounds PowerPoint Presentation ID2068191 Are Ionic Bonds Metal And Nonmetal The periodic table can help us. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. Ionic bonds are formed between cations and anions. a compound that contains ions and is held together by ionic bonds is called an ionic compound. A cation is formed when a metal ion loses. Are Ionic Bonds Metal And Nonmetal.
From www.worksheetsplanet.com
What is an Ionic Bond? Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. binary ionic compounds are composed of just two elements: A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. a compound that contains ions and is held together by. Are Ionic Bonds Metal And Nonmetal.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Bonds Metal And Nonmetal ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. A metal (which forms the cations) and a nonmetal (which forms the. a compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us. By definition,. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Chemical Bonding. ppt download Are Ionic Bonds Metal And Nonmetal A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. The periodic table can help us. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. a compound that contains ions and is held together by ionic bonds is called. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Ionic/Covalent/Metallic Bonding Notes ppt download Are Ionic Bonds Metal And Nonmetal The periodic table can help us. A metal (which forms the cations) and a nonmetal (which forms the. binary ionic compounds are composed of just two elements: A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. a compound that contains ions and is held together by ionic. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Bonding Ionic bond (formula units) Between metal and a nonmetal ppt download Are Ionic Bonds Metal And Nonmetal A metal (which forms the cations) and a nonmetal (which forms the. The periodic table can help us. a compound that contains ions and is held together by ionic bonds is called an ionic compound. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. binary. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Chemistry IANB Page 11 PowerPoint Presentation, free download ID3818525 Are Ionic Bonds Metal And Nonmetal Ionic bonds are formed between cations and anions. a compound that contains ions and is held together by ionic bonds is called an ionic compound. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. binary ionic compounds are composed of just two elements: A metal (which forms. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Bonding Ionic bond (formula units) Between metal and a nonmetal ppt download Are Ionic Bonds Metal And Nonmetal Ionic bonds are formed between cations and anions. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. a compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us. binary ionic compounds are composed. Are Ionic Bonds Metal And Nonmetal.
From stock.adobe.com
Ionic bond and electrostatic attraction from chemical bonding outline diagram. Labeled Are Ionic Bonds Metal And Nonmetal a compound that contains ions and is held together by ionic bonds is called an ionic compound. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion loses a valence electron while an anion. Are Ionic Bonds Metal And Nonmetal.
From slidetodoc.com
Unit 8 Covalent Bonding To bond or not Are Ionic Bonds Metal And Nonmetal a compound that contains ions and is held together by ionic bonds is called an ionic compound. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. ionic bonding. Are Ionic Bonds Metal And Nonmetal.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Bonds Metal And Nonmetal Ionic bonds are formed between cations and anions. a compound that contains ions and is held together by ionic bonds is called an ionic compound. A metal (which forms the cations) and a nonmetal (which forms the. The periodic table can help us. binary ionic compounds are composed of just two elements: By definition, a metal is relatively. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT ATOMS PowerPoint Presentation, free download ID2618827 Are Ionic Bonds Metal And Nonmetal a compound that contains ions and is held together by ionic bonds is called an ionic compound. A metal (which forms the cations) and a nonmetal (which forms the. The periodic table can help us. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. By definition, a metal. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Ionic Bonding PowerPoint Presentation, free download ID2435173 Are Ionic Bonds Metal And Nonmetal Ionic bonds are formed between cations and anions. a compound that contains ions and is held together by ionic bonds is called an ionic compound. binary ionic compounds are composed of just two elements: By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. The periodic table can help. Are Ionic Bonds Metal And Nonmetal.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Are Ionic Bonds Metal And Nonmetal A metal (which forms the cations) and a nonmetal (which forms the. binary ionic compounds are composed of just two elements: a compound that contains ions and is held together by ionic bonds is called an ionic compound. Ionic bonds are formed between cations and anions. ionic bonding results in compounds known as ionic, or electrovalent, compounds,. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Chapter 5 Lecture presentation ppt download Are Ionic Bonds Metal And Nonmetal The periodic table can help us. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free download ID6776451 Are Ionic Bonds Metal And Nonmetal a compound that contains ions and is held together by ionic bonds is called an ionic compound. Ionic bonds are formed between cations and anions. The periodic table can help us. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. A metal (which forms the cations). Are Ionic Bonds Metal And Nonmetal.
From learnwithdrscott.com
Ionic Bond Definition Easy Hard Science Are Ionic Bonds Metal And Nonmetal a compound that contains ions and is held together by ionic bonds is called an ionic compound. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. binary ionic. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Chapter 10 Molecular Structure Liquids and Solids ppt download Are Ionic Bonds Metal And Nonmetal A metal (which forms the cations) and a nonmetal (which forms the. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. The periodic table can help us. binary ionic compounds are composed of just two elements: By definition, a metal. Are Ionic Bonds Metal And Nonmetal.
From slidetodoc.com
Chemistry 100 Chapter 10 Chemical Bonding Chemical Bonds Are Ionic Bonds Metal And Nonmetal Ionic bonds are formed between cations and anions. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. a compound that contains ions and is held together by ionic bonds is called an ionic compound. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Chapter 20 Section ppt download Are Ionic Bonds Metal And Nonmetal ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. The periodic table can help us. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. A metal (which forms the cations) and a nonmetal (which forms the.. Are Ionic Bonds Metal And Nonmetal.
From slidetodoc.com
Chemical Bonds Chemical Bonds An attractive force that Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. Ionic bonds are formed between cations and anions. binary ionic compounds are composed of just two elements: The periodic table can help us. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by. Are Ionic Bonds Metal And Nonmetal.
From www.studocu.com
Chemical bonds General Chemistry Chemical bonds ionic metal and nonmetal covalent two or Are Ionic Bonds Metal And Nonmetal The periodic table can help us. A metal (which forms the cations) and a nonmetal (which forms the. Ionic bonds are formed between cations and anions. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. binary ionic compounds are composed of just two elements: a compound that. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Noble Gases and Valence e Ionization Energy and Bonding PowerPoint Presentation ID5875405 Are Ionic Bonds Metal And Nonmetal A metal (which forms the cations) and a nonmetal (which forms the. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. a compound that contains ions and is held together by ionic bonds is called an ionic compound. By definition, a metal is relatively stable if. Are Ionic Bonds Metal And Nonmetal.
From diagramdataperianths.z21.web.core.windows.net
Diagram Ionic Bond Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. The periodic table can help us. Ionic bonds are formed between cations and anions. a compound that contains ions and. Are Ionic Bonds Metal And Nonmetal.
From www.youtube.com
Ionic bonds Reaction of metals & Nonmetals Metals and non metals Chemistry Khan Academy Are Ionic Bonds Metal And Nonmetal A metal (which forms the cations) and a nonmetal (which forms the. By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. Ionic bonds are formed between cations and anions. The. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT NonMetals PowerPoint Presentation, free download ID2409577 Are Ionic Bonds Metal And Nonmetal A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. a compound that contains ions and is held together by ionic bonds is called an ionic compound. A metal (which forms the cations) and a nonmetal (which forms the. Ionic bonds are formed between cations and anions. By definition,. Are Ionic Bonds Metal And Nonmetal.
From slidetodoc.com
BASIC CHEMISTRY Chapter 2 Advanced Human Anatomy Introduction Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. A cation is formed when a metal ion loses a valence electron while an anion is formed when a. Are Ionic Bonds Metal And Nonmetal.
From studynympholept.z21.web.core.windows.net
A Covalent Bond Forms Between Two Metals Are Ionic Bonds Metal And Nonmetal binary ionic compounds are composed of just two elements: a compound that contains ions and is held together by ionic bonds is called an ionic compound. A metal (which forms the cations) and a nonmetal (which forms the. Ionic bonds are formed between cations and anions. The periodic table can help us. ionic bonding results in compounds. Are Ionic Bonds Metal And Nonmetal.
From slidetodoc.com
Bonding Between Atoms Why Do Atoms Form Bonds Are Ionic Bonds Metal And Nonmetal binary ionic compounds are composed of just two elements: A metal (which forms the cations) and a nonmetal (which forms the. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the. Are Ionic Bonds Metal And Nonmetal.
From slideplayer.com
Ch. 7 Ionic Bonds. ppt download Are Ionic Bonds Metal And Nonmetal By definition, a metal is relatively stable if it loses electrons to form a complete valence shell and becomes. The periodic table can help us. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals. Ionic bonds are formed between cations and anions. a compound that contains. Are Ionic Bonds Metal And Nonmetal.
From www.slideserve.com
PPT Chapter 1 Chemical Bonding PowerPoint Presentation, free download ID1482753 Are Ionic Bonds Metal And Nonmetal binary ionic compounds are composed of just two elements: a compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us. A cation is formed when a metal ion loses a valence electron while an anion is formed when a non. Ionic bonds are formed between cations. Are Ionic Bonds Metal And Nonmetal.