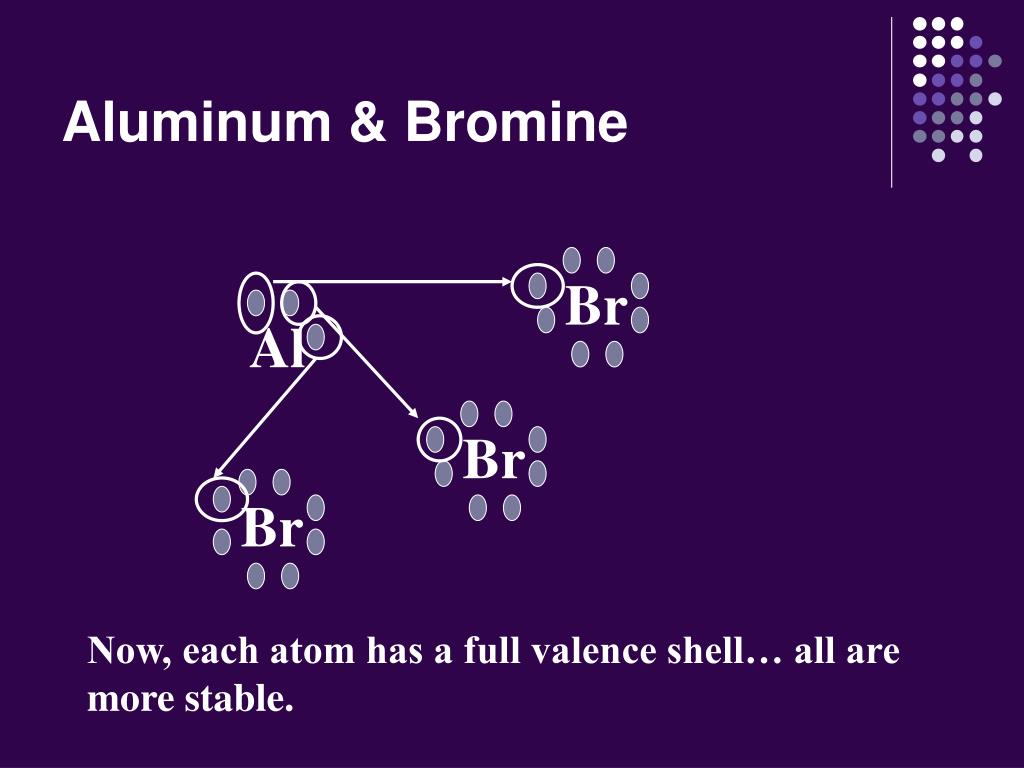
Bromine Bonding Properties . Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Sources, facts, uses, scarcity (sri), podcasts,. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. The strongest bond for a homonuclear diatomic. Bromine is soluble in water and organic solvents. it has properties that are intermediate between iodine and chlorine. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds.
from www.slideserve.com
Sources, facts, uses, scarcity (sri), podcasts,. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. The strongest bond for a homonuclear diatomic. it has properties that are intermediate between iodine and chlorine. Bromine is soluble in water and organic solvents.
PPT Chemistry Unit 2 PowerPoint Presentation, free download ID3668529
Bromine Bonding Properties Sources, facts, uses, scarcity (sri), podcasts,. The strongest bond for a homonuclear diatomic. it has properties that are intermediate between iodine and chlorine. Bromine is soluble in water and organic solvents. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. Sources, facts, uses, scarcity (sri), podcasts,.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Bonding Properties bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine is soluble in water and organic solvents. The strongest bond for a homonuclear diatomic. Sources, facts, uses, scarcity (sri), podcasts,. it. Bromine Bonding Properties.
From www.pw.live
Bromine Formula, Valency, Mass And Properties Bromine Bonding Properties The strongest bond for a homonuclear diatomic. it has properties that are intermediate between iodine and chlorine. Sources, facts, uses, scarcity (sri), podcasts,. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. bromine, a halogen element with the symbol br, interacts. Bromine Bonding Properties.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Bonding Properties bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. it has properties that are intermediate between iodine and chlorine. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Sources,. Bromine Bonding Properties.
From dxonccmlx.blob.core.windows.net
Bromine Group Properties at James Carr blog Bromine Bonding Properties bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Sources, facts, uses, scarcity (sri), podcasts,. bromine, a halogen element with the symbol br, interacts with various elements. Bromine Bonding Properties.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Bonding Properties Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. it has properties that are intermediate between iodine and chlorine. Bromine is soluble in water and organic solvents. Sources, facts, uses, scarcity (sri), podcasts,. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine. Bromine Bonding Properties.
From www.slideserve.com
PPT Bromine ! PowerPoint Presentation, free download ID2812995 Bromine Bonding Properties bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. it has properties that are intermediate between iodine and chlorine. The. Bromine Bonding Properties.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Bonding Properties it has properties that are intermediate between iodine and chlorine. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine is soluble in water and organic solvents. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than.. Bromine Bonding Properties.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Bonding Properties Bromine is soluble in water and organic solvents. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Sources, facts, uses, scarcity (sri), podcasts,. The strongest bond for a homonuclear diatomic. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine. Bromine Bonding Properties.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Bonding Properties Bromine is soluble in water and organic solvents. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. The strongest bond for a homonuclear diatomic. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine bond energies tend to be lower. Bromine Bonding Properties.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Bonding Properties bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. The strongest bond for a homonuclear diatomic. it has properties that are intermediate between iodine and chlorine. bromine, a halogen element with the symbol br, interacts with various elements and compounds to. Bromine Bonding Properties.
From cms.gutow.uwosh.edu
Bromine Bromine Bonding Properties The strongest bond for a homonuclear diatomic. Sources, facts, uses, scarcity (sri), podcasts,. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. Bromine is soluble in water and organic solvents. it has properties that are intermediate between iodine and chlorine. Bromine atoms. Bromine Bonding Properties.
From www.numerade.com
SOLVED Below, draw and label an atomic/molecular level picture of the Bromine Bonding Properties Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. it has properties that are intermediate between iodine and chlorine. Bromine is soluble in water and organic solvents. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than.. Bromine Bonding Properties.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Bonding Properties The strongest bond for a homonuclear diatomic. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine. Bromine Bonding Properties.
From www.chemistrylearner.com
Bromine Facts, Symbol, Discovery, Properties, Uses Bromine Bonding Properties bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. The strongest bond for a homonuclear diatomic. Sources, facts, uses, scarcity (sri), podcasts,. Bromine is soluble in water and organic solvents. it has properties that are intermediate between iodine and chlorine. Bromine atoms. Bromine Bonding Properties.
From www.researchgate.net
Results of the C2/m structure of bromine. (a) C2/m structure model Bromine Bonding Properties Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. it has properties that are intermediate between iodine and chlorine. Bromine is soluble in water and organic solvents.. Bromine Bonding Properties.
From dxozlxzjl.blob.core.windows.net
Bromine And Chemical Symbol at Mildred Lyle blog Bromine Bonding Properties it has properties that are intermediate between iodine and chlorine. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. The strongest bond for a homonuclear diatomic. bromine, a halogen element with the symbol br, interacts with various elements and compounds to. Bromine Bonding Properties.
From chemicaldb.netlify.app
What is a chemical property of bromine Bromine Bonding Properties it has properties that are intermediate between iodine and chlorine. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Bromine is soluble in water and organic solvents. Sources, facts, uses, scarcity (sri), podcasts,. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine. Bromine Bonding Properties.
From exoqkqwbx.blob.core.windows.net
What Properties Does Bromine Have at Glenn Buckley blog Bromine Bonding Properties bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Sources, facts, uses, scarcity (sri), podcasts,. The strongest bond for a homonuclear diatomic. bromine, a halogen element with. Bromine Bonding Properties.
From www.nuclear-power.com
What is Bromine Properties of Bromine Element Symbol Br nuclear Bromine Bonding Properties Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. it has properties that are intermediate between iodine and chlorine. The strongest bond for a homonuclear diatomic. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. bromine bond energies tend. Bromine Bonding Properties.
From www.britannica.com
bromine Properties, Uses, & Facts Britannica Bromine Bonding Properties Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. it has properties that are intermediate between iodine and chlorine. Bromine is soluble in water and organic solvents. The strongest bond for a homonuclear diatomic. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range. Bromine Bonding Properties.
From www.istockphoto.com
Br Bromine Element Information Facts Properties Trends Uses And Bromine Bonding Properties Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. The strongest bond for a homonuclear diatomic. Bromine is soluble in water and organic solvents. Sources, facts, uses, scarcity (sri), podcasts,. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing. Bromine Bonding Properties.
From matmake.com
Bromine Properties Bromine Bonding Properties it has properties that are intermediate between iodine and chlorine. Bromine is soluble in water and organic solvents. bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine bond energies. Bromine Bonding Properties.
From www.slideserve.com
PPT Ch. 7 and 8 Bonding PowerPoint Presentation, free download ID Bromine Bonding Properties Bromine is soluble in water and organic solvents. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. The strongest bond for a homonuclear diatomic. Sources, facts, uses, scarcity. Bromine Bonding Properties.
From www.slideserve.com
PPT Chemistry Unit 2 PowerPoint Presentation, free download ID3668529 Bromine Bonding Properties The strongest bond for a homonuclear diatomic. it has properties that are intermediate between iodine and chlorine. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. Sources, facts, uses, scarcity (sri), podcasts,. bromine, a halogen element with the symbol br, interacts. Bromine Bonding Properties.
From www.slideserve.com
PPT Bromine PowerPoint Presentation, free download ID2319111 Bromine Bonding Properties The strongest bond for a homonuclear diatomic. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. it has properties that are intermediate between iodine and chlorine. Sources, facts, uses, scarcity (sri), podcasts,. Bromine is soluble in water and organic solvents. Bromine atoms. Bromine Bonding Properties.
From www.slideshare.net
Bromine berry Bromine Bonding Properties The strongest bond for a homonuclear diatomic. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. it has properties that are intermediate between iodine and chlorine. Bromine. Bromine Bonding Properties.
From studiousguy.com
Bromine (Br) Properties & Uses StudiousGuy Bromine Bonding Properties bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Bromine is soluble in water and organic solvents. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. The strongest bond for a homonuclear diatomic. bromine bond energies tend to be lower. Bromine Bonding Properties.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Bromine Bonding Properties bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. it has properties that are intermediate between iodine and chlorine. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. The. Bromine Bonding Properties.
From techschematic.com
Understanding the Electron Dot Diagram for Bromine A Visual Bromine Bonding Properties bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Sources, facts, uses, scarcity (sri), podcasts,. Bromine is soluble in water and organic solvents. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing. Bromine Bonding Properties.
From lets-talk-bromine.bsef.com
Periodic table Have you heard of these 5 elements? Let's talk bromine Bromine Bonding Properties bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Bromine is soluble in water and organic solvents. it has properties that are intermediate between iodine and chlorine. Sources, facts, uses, scarcity (sri), podcasts,. The strongest bond for a homonuclear diatomic. bromine bond energies tend to. Bromine Bonding Properties.
From www.youtube.com
Br2 Lewis Structure How to Draw the Lewis Dot Structure for Dibromine Bromine Bonding Properties bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker. Bromine Bonding Properties.
From thechemistrynotes.com
Bromine (Br) Element Properties and Amazing Uses, Facts Bromine Bonding Properties bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. it has properties that are intermediate between iodine and chlorine. Sources, facts, uses, scarcity (sri), podcasts,. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is. Bromine Bonding Properties.
From www.researchgate.net
(a) Electronic properties of iodine, bromine, and chlorine; (b Bromine Bonding Properties bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. The strongest bond for a homonuclear diatomic. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than. it has properties that. Bromine Bonding Properties.
From www.showme.com
Sulfur Bromine Covalent Bonding Science ShowMe Bromine Bonding Properties bromine, a halogen element with the symbol br, interacts with various elements and compounds to form a range of bromine compounds. it has properties that are intermediate between iodine and chlorine. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. Sources, facts, uses, scarcity (sri), podcasts,. Bromine is soluble in water and. Bromine Bonding Properties.
From slideplayer.com
Types of reactions Chapter ppt download Bromine Bonding Properties it has properties that are intermediate between iodine and chlorine. Bromine is soluble in water and organic solvents. Bromine atoms are highly electronegative, with an electronegativity of about 2.96 on the pauling scale. bromine bond energies tend to be lower than chlorine bond energies but higher than iodine bond energies, and bromine is a weaker oxidizing agent than.. Bromine Bonding Properties.