Heat Capacity Of Calorimeter Combustion . heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. How much heat was produced by the. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound.
from www.chegg.com
heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. How much heat was produced by the. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of.
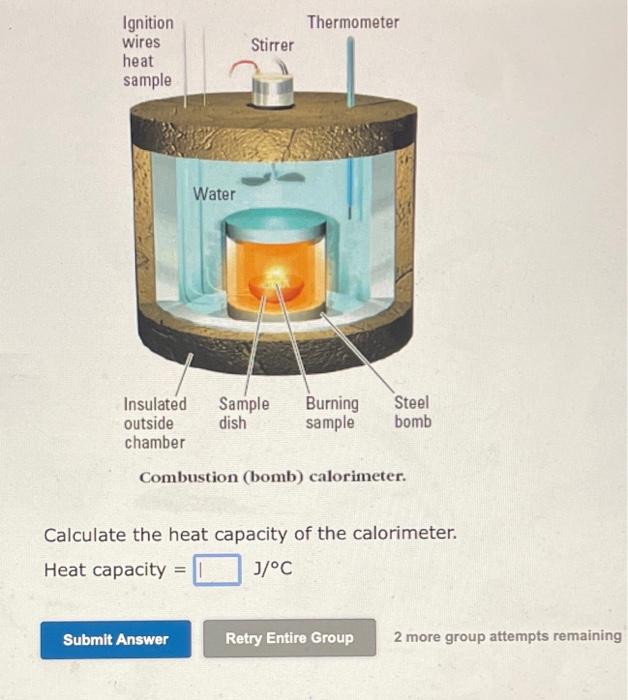
Solved Combustion (bomb) calorimeter. Calculate the heat
Heat Capacity Of Calorimeter Combustion heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. How much heat was produced by the. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Heat Capacity Of Calorimeter Combustion heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. How much heat was produced by the. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). heat capacity is defined as the amount of heat needed to increase. Heat Capacity Of Calorimeter Combustion.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Heat Capacity Of Calorimeter Combustion to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. heats of combustion are. Heat Capacity Of Calorimeter Combustion.
From www.chegg.com
Solved At constant volume, the heat of combustion of a Heat Capacity Of Calorimeter Combustion the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of. Heat Capacity Of Calorimeter Combustion.
From www.chegg.com
Solved Calculate the heat capacity of a calorimeter if the Heat Capacity Of Calorimeter Combustion heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. How much heat was produced by the. heat capacity of the calorimeter. Heat Capacity Of Calorimeter Combustion.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity Of Calorimeter Combustion where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. heat capacity is defined as the amount of heat needed to increase the temperature of. Heat Capacity Of Calorimeter Combustion.
From exosbbnfj.blob.core.windows.net
Calorimetry Ap Chemistry at Michael Faust blog Heat Capacity Of Calorimeter Combustion the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. where q is the amount. Heat Capacity Of Calorimeter Combustion.
From www.scribd.com
B. Heat Capacity of Calorimeter PDF Heat Capacity Of Calorimeter Combustion heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of. Heat Capacity Of Calorimeter Combustion.
From www.numerade.com
SOLVED Use the References to access important values if needed for Heat Capacity Of Calorimeter Combustion to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c.. Heat Capacity Of Calorimeter Combustion.
From chemistryvce.weebly.com
Measuring energy VCE Chemistry Heat Capacity Of Calorimeter Combustion How much heat was produced by the. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). heat capacity of the calorimeter system can be determined, allowing. Heat Capacity Of Calorimeter Combustion.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Heat Capacity Of Calorimeter Combustion the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. heat capacity. Heat Capacity Of Calorimeter Combustion.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Heat Capacity Of Calorimeter Combustion heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. How much heat was produced by the. the combustion of 1 mole of. Heat Capacity Of Calorimeter Combustion.
From slidetodoc.com
BOMB CALORIMETRY Heat Internal Energy and Enthalpy Heat Heat Capacity Of Calorimeter Combustion How much heat was produced by the. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. heats of combustion are most common, in which the combustible material is explosively burned. Heat Capacity Of Calorimeter Combustion.
From www.coursehero.com
[Solved] Heat capacity of the calorimeter?. A bomb calorimeter, or Heat Capacity Of Calorimeter Combustion the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1.. Heat Capacity Of Calorimeter Combustion.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Heat Capacity Of Calorimeter Combustion heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard. Heat Capacity Of Calorimeter Combustion.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Heat Capacity Of Calorimeter Combustion heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). where q is the amount of heat according to the change in temperature measured in joules. Heat Capacity Of Calorimeter Combustion.
From www.toppr.com
Calculate the heat of combustion of methane at constant volume. The Heat Capacity Of Calorimeter Combustion where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. How much heat was produced by the. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heat capacity is defined as the amount. Heat Capacity Of Calorimeter Combustion.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Heat Capacity Of Calorimeter Combustion heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. . Heat Capacity Of Calorimeter Combustion.
From www.numerade.com
SOLVED A bomb calorimeter or a constant volume calorimeler; is a Heat Capacity Of Calorimeter Combustion How much heat was produced by the. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. heat capacity of the calorimeter system can be determined, allowing for the calculation. Heat Capacity Of Calorimeter Combustion.
From www.numerade.com
SOLVEDThe heat capacity of a bomb calorimeter was determined by Heat Capacity Of Calorimeter Combustion heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. to. Heat Capacity Of Calorimeter Combustion.
From www.slideserve.com
PPT Introduction to Thermochemistry PowerPoint Presentation, free Heat Capacity Of Calorimeter Combustion heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. heat capacity is defined as the amount of heat needed to increase the temperature of the entire calorimeter by 1. How much. Heat Capacity Of Calorimeter Combustion.
From 2012books.lardbucket.org
Calorimetry Heat Capacity Of Calorimeter Combustion to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. where q is the amount of heat according to the change in temperature measured in joules and cv is the. Heat Capacity Of Calorimeter Combustion.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Heat Capacity Of Calorimeter Combustion the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass. Heat Capacity Of Calorimeter Combustion.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity Of Calorimeter Combustion the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. where q is the amount of heat according. Heat Capacity Of Calorimeter Combustion.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube Heat Capacity Of Calorimeter Combustion to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. the calorimeter contains 775 g of water, and the bomb itself has a heat. Heat Capacity Of Calorimeter Combustion.
From slideplayer.com
Chapter 5 Thermochemistry ppt download Heat Capacity Of Calorimeter Combustion where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. How much heat was produced by the. heats of combustion are most common, in which. Heat Capacity Of Calorimeter Combustion.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Heat Capacity Of Calorimeter Combustion to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. the calorimeter contains. Heat Capacity Of Calorimeter Combustion.
From www.numerade.com
SOLVED A bomb calorimeter, or constant volume calorimeter; is a device Heat Capacity Of Calorimeter Combustion the calorimeter contains 775 g of water, and the bomb itself has a heat capacity of 893 j/°c. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the. Heat Capacity Of Calorimeter Combustion.
From www.numerade.com
SOLVEDUse the References to access important values if needed for this Heat Capacity Of Calorimeter Combustion to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of.. Heat Capacity Of Calorimeter Combustion.
From ar.inspiredpencil.com
Heat Of Combustion Lab Heat Capacity Of Calorimeter Combustion the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. to measure. Heat Capacity Of Calorimeter Combustion.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Heat Capacity Of Calorimeter Combustion to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). heat capacity is defined as the amount of heat needed to increase the temperature of the entire. Heat Capacity Of Calorimeter Combustion.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Heat Capacity Of Calorimeter Combustion where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heat capacity of the calorimeter system can be determined, allowing for the calculation of. Heat Capacity Of Calorimeter Combustion.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity Of Calorimeter Combustion heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases $\pu{2.82\times10^3 kj}$ of heat. to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. heat capacity of. Heat Capacity Of Calorimeter Combustion.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Heat Capacity Of Calorimeter Combustion where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. heat capacity of the calorimeter system can be determined, allowing for the calculation of the heat of. How much heat was produced by the. the combustion of 1 mole of glucose $\ce{c6h12o6}$ releases. Heat Capacity Of Calorimeter Combustion.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Heat Capacity Of Calorimeter Combustion heats of combustion are most common, in which the combustible material is explosively burned in a strong, steel container (the “bomb”). where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. to measure the heat capacity of the calorimeter, we first burn a. Heat Capacity Of Calorimeter Combustion.
From www.chegg.com
Solved Combustion (bomb) calorimeter. Calculate the heat Heat Capacity Of Calorimeter Combustion to measure the heat capacity of the calorimeter, we first burn a carefully weighed mass of a standard compound. where q is the amount of heat according to the change in temperature measured in joules and cv is the heat capacity of the. heat capacity is defined as the amount of heat needed to increase the temperature. Heat Capacity Of Calorimeter Combustion.