What Is Threshold Frequency Binding Energy . Work function (w) is the energy required to remove an electron from the material’s surface. In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. The threshold frequency of the metal is x×1014sec−1, where x (approx.) is: There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. The threshold frequency refers to the. The threshold frequency is defined as: Threshold frequency (f 0) is the minimum frequency of incident light. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal.
from www.numerade.com
The threshold frequency of the metal is x×1014sec−1, where x (approx.) is: Work function (w) is the energy required to remove an electron from the material’s surface. If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: Threshold frequency (f 0) is the minimum frequency of incident light. The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. The threshold frequency refers to the. The threshold frequency is defined as: The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an.
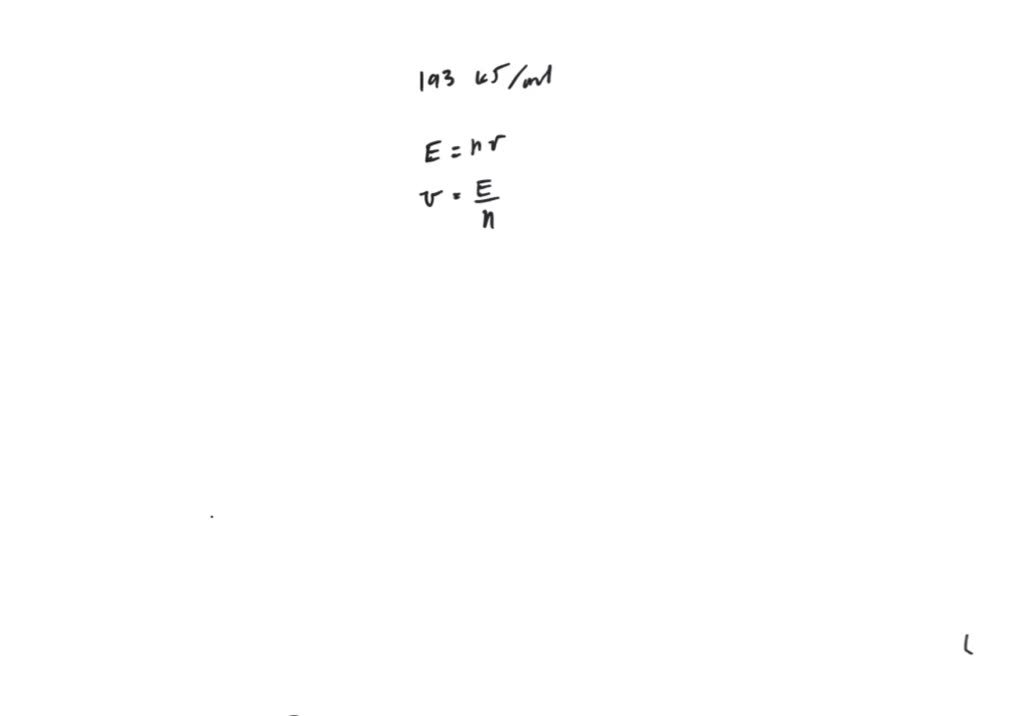
The binding energy of electrons in a metal is 193 kJ/mol. Find the
What Is Threshold Frequency Binding Energy There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. Threshold frequency (f 0) is the minimum frequency of incident light. The threshold frequency is defined as: The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Work function (w) is the energy required to remove an electron from the material’s surface. If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. The threshold frequency refers to the. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? The threshold frequency of the metal is x×1014sec−1, where x (approx.) is: In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an.
From www.tessshebaylo.com
Binding Energy Wavelength Equation Tessshebaylo What Is Threshold Frequency Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons. What Is Threshold Frequency Binding Energy.
From www.toppr.com
Calculate the binding energy per mole when threshold wavelength is 240 nm. What Is Threshold Frequency Binding Energy The threshold frequency of the metal is x×1014sec−1, where x (approx.) is: What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: The threshold frequency refers to the. The. What Is Threshold Frequency Binding Energy.
From www.youtube.com
Calculate the binding energy per mole when threshold wavelength of What Is Threshold Frequency Binding Energy The threshold frequency refers to the. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? The threshold frequency is defined as: If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: The minimum frequency of incident electromagnetic radiation. What Is Threshold Frequency Binding Energy.
From www.numerade.com
The binding energy of electrons in a metal is 193 kJ / mol . Find the What Is Threshold Frequency Binding Energy The threshold frequency is defined as: In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? There is a threshold frequency below which no electrons are ejected, because the individual photon interacting. What Is Threshold Frequency Binding Energy.
From pressbooks.online.ucf.edu
Nuclear Binding Energy University Physics Volume 3 What Is Threshold Frequency Binding Energy The threshold frequency is defined as: The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: Work function (w) is the energy required to remove an. What Is Threshold Frequency Binding Energy.
From www.numerade.com
SOLVEDThe binding energy of electrons in a metal is 193 kjimol. Find What Is Threshold Frequency Binding Energy The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. Work function (w) is the energy required to remove an electron from the material’s surface. There is a threshold. What Is Threshold Frequency Binding Energy.
From www.toppr.com
The work function of a photoelectric material is 3.3 eV. It threshold What Is Threshold Frequency Binding Energy There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? Work function (w) is the energy required to remove an electron from the material’s surface. The threshold frequency. What Is Threshold Frequency Binding Energy.
From www.youtube.com
Calculate the binding energy per mole when threshold wavelength of What Is Threshold Frequency Binding Energy The threshold frequency is defined as: The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. The threshold frequency of the metal is x×1014sec−1, where x (approx.) is:. What Is Threshold Frequency Binding Energy.
From courses.lumenlearning.com
The Photoelectric Effect Physics What Is Threshold Frequency Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an.. What Is Threshold Frequency Binding Energy.
From www.youtube.com
CHEM 101 Photoelectric Effect Threshold Frequency and Binding Energy What Is Threshold Frequency Binding Energy In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? The threshold frequency is defined as: The binding energy (be) of a nucleus is the energy needed to separate it into individual. What Is Threshold Frequency Binding Energy.
From www.youtube.com
The threshold wavelength for emission of electrons from a given metal What Is Threshold Frequency Binding Energy There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. Threshold frequency (f 0) is the minimum frequency of incident light. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? The threshold frequency is defined as the. What Is Threshold Frequency Binding Energy.
From www.chegg.com
Solved The binding energy of electrons in a metal is 193 What Is Threshold Frequency Binding Energy There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. Threshold frequency (f 0) is the minimum frequency of incident light. In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. If the binding energy of electrons in a metal is 250. What Is Threshold Frequency Binding Energy.
From www.slideserve.com
PPT How to Get the Binding Energy by Mass Spectrometry PowerPoint What Is Threshold Frequency Binding Energy The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. The threshold frequency of the metal is x×1014sec−1, where x (approx.) is: The threshold frequency is defined as: If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the. What Is Threshold Frequency Binding Energy.
From www.coursehero.com
[Solved] What is the binding energy of an electron in a metal whose What Is Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. Work function (w) is the energy required to remove. What Is Threshold Frequency Binding Energy.
From www.tessshebaylo.com
Binding Energy Wavelength Equation Tessshebaylo What Is Threshold Frequency Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. The threshold frequency is. What Is Threshold Frequency Binding Energy.
From www.researchgate.net
Binding energy shift of core level and valence band states upon What Is Threshold Frequency Binding Energy The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. The threshold frequency refers to the. Threshold frequency (f 0) is the minimum frequency of incident light. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? In this example, we. What Is Threshold Frequency Binding Energy.
From www.numerade.com
The binding energy of electrons in a metal is 193 kJ/mol. Find the What Is Threshold Frequency Binding Energy In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. What is the binding. What Is Threshold Frequency Binding Energy.
From www.slideserve.com
PPT 1.2 Radiation and Quantum Phenomena Quantum What Is Threshold Frequency Binding Energy Work function (w) is the energy required to remove an electron from the material’s surface. The threshold frequency is defined as: The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz?. What Is Threshold Frequency Binding Energy.
From www.youtube.com
CHEMISTRY 101 Photoelectric Effect, Threshold Frequency YouTube What Is Threshold Frequency Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Threshold frequency (f 0) is the minimum frequency of incident light. Work function (w) is the energy required to remove an electron from the material’s surface. There is a threshold frequency below which no electrons are ejected, because the individual photon. What Is Threshold Frequency Binding Energy.
From www.toppr.com
CHEMISTRY e threshold frequency of the striking If the binding energy What Is Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. Work function (w) is the energy required to remove an electron from the material’s surface. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? There is a threshold frequency below which no electrons are ejected, because the. What Is Threshold Frequency Binding Energy.
From www.shutterstock.com
Nuclear Binding Energy Curve Graph Binding Stock Illustration What Is Threshold Frequency Binding Energy The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with. What Is Threshold Frequency Binding Energy.
From eduinput.com
What is Binding Energy?Definition, Types, And Applications What Is Threshold Frequency Binding Energy The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. In this. What Is Threshold Frequency Binding Energy.
From www.manminchurch.se
asistenţă Baie impuls calculate threshold frequency practică Retenţie sân What Is Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. If the binding energy. What Is Threshold Frequency Binding Energy.
From www.youtube.com
HTPIB30O The Curve of Binding Energy YouTube What Is Threshold Frequency Binding Energy The threshold frequency refers to the. The threshold frequency is defined as: What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? Work function (w) is the energy required to remove an electron from the material’s surface. There is a threshold frequency below which no electrons are ejected, because. What Is Threshold Frequency Binding Energy.
From www.youtube.com
Calculate the threshold frequency of metal if the binding energy is What Is Threshold Frequency Binding Energy The threshold frequency is defined as: In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or. What Is Threshold Frequency Binding Energy.
From www.chegg.com
Solved What Is The Binding Energy (in J/mol Or KJ/mol) Of... What Is Threshold Frequency Binding Energy If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons.. What Is Threshold Frequency Binding Energy.
From www.researchgate.net
Binding energy and inelastic loss in the bb channel The below What Is Threshold Frequency Binding Energy The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. Threshold frequency (f 0) is the minimum frequency of incident light. There is a threshold frequency below which no electrons are ejected, because the individual. What Is Threshold Frequency Binding Energy.
From www.tessshebaylo.com
Binding Energy Wavelength Equation Tessshebaylo What Is Threshold Frequency Binding Energy The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission of electrons is not possible. The threshold frequency is defined as: The binding energy (be) of a nucleus is the energy needed to. What Is Threshold Frequency Binding Energy.
From www.researchgate.net
Intramolecular contribution to the polaron binding energy. 1 , 13 , 46 What Is Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. The threshold frequency refers to the. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. In this example, we calculate the. What Is Threshold Frequency Binding Energy.
From www.chegg.com
Solved 4. The binding energy of Ni is 3.017x1024 eVmol1. a) What Is Threshold Frequency Binding Energy The threshold frequency of the metal is x×1014sec−1, where x (approx.) is: Threshold frequency (f 0) is the minimum frequency of incident light. The minimum frequency of incident electromagnetic radiation required to remove a photoelectron from the surface of a metal. The threshold frequency is defined as: There is a threshold frequency below which no electrons are ejected, because the. What Is Threshold Frequency Binding Energy.
From www.researchgate.net
Exciton binding energies measurement Temperaturedependent PL spectra What Is Threshold Frequency Binding Energy There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. Threshold frequency (f 0) is the minimum frequency of incident light. The threshold frequency is defined as: The binding energy (be) of a nucleus is the energy needed to separate it into individual protons and neutrons. Work function (w). What Is Threshold Frequency Binding Energy.
From www.britannica.com
Nuclear binding energy Definition, Formula, Mass Defect, & Graph What Is Threshold Frequency Binding Energy Threshold frequency (f 0) is the minimum frequency of incident light. Work function (w) is the energy required to remove an electron from the material’s surface. The threshold frequency is defined as: The threshold frequency of the metal is x×1014sec−1, where x (approx.) is: The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric. What Is Threshold Frequency Binding Energy.
From www.toppr.com
The threshold frequency of metal the binding energy is 198.9 KJ mol1 What Is Threshold Frequency Binding Energy In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. The threshold frequency of the metal is x×1014sec−1, where x (approx.) is: What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? Threshold frequency (f 0) is the minimum frequency of incident. What Is Threshold Frequency Binding Energy.
From www.slideserve.com
PPT Binding energy in atoms and nuclei PowerPoint Presentation, free What Is Threshold Frequency Binding Energy In this example, we calculate the threshold frequency and binding energy from the kinetic energy of an. Threshold frequency (f 0) is the minimum frequency of incident light. There is a threshold frequency below which no electrons are ejected, because the individual photon interacting with an individual electron has. The threshold frequency refers to the. What is the binding energy. What Is Threshold Frequency Binding Energy.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition What Is Threshold Frequency Binding Energy What is the binding energy of an electron in a metal whose threshold frequency for photoelectrons is 2.50 × 1014 hz? If the binding energy of electrons in a metal is 250 kjmol−1 then, the threshold frequency of the metal is: The threshold frequency is defined as the minimum frequency of the incident radiation below which photoelectric emission or emission. What Is Threshold Frequency Binding Energy.